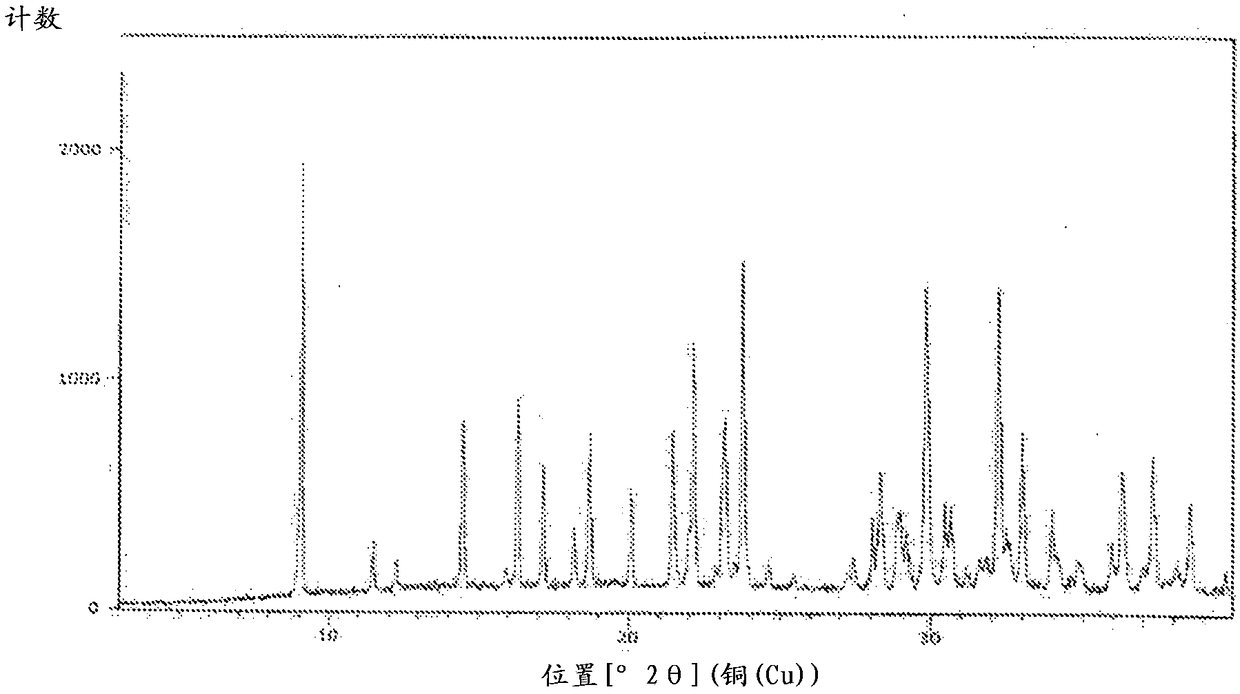
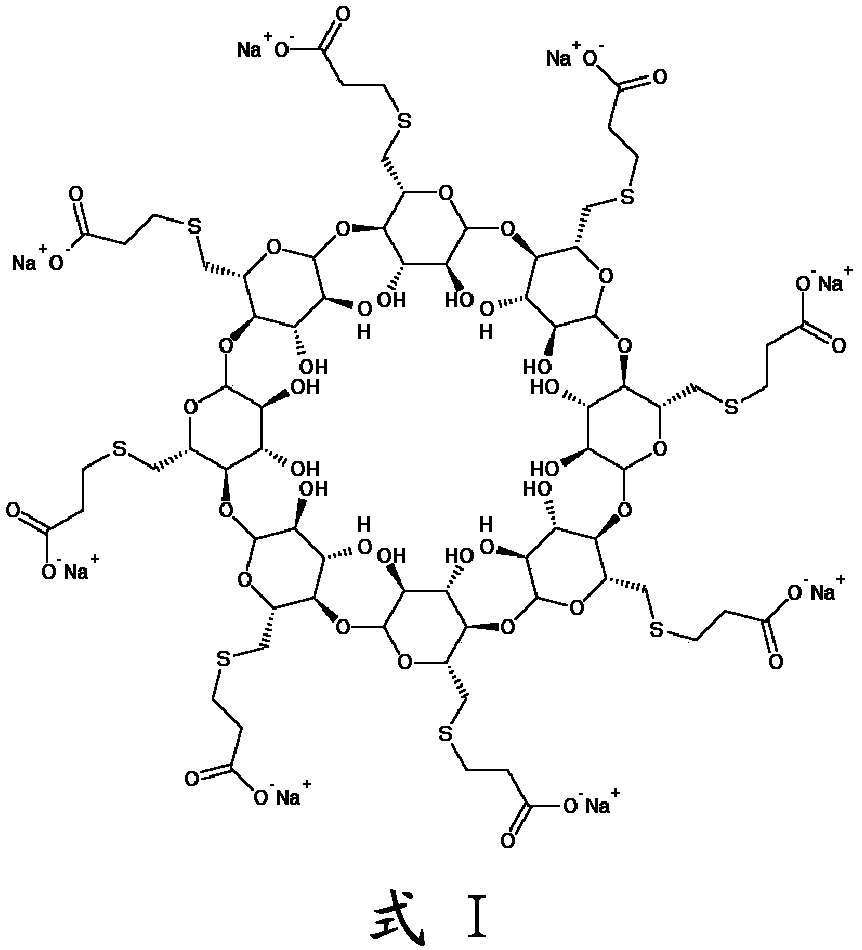
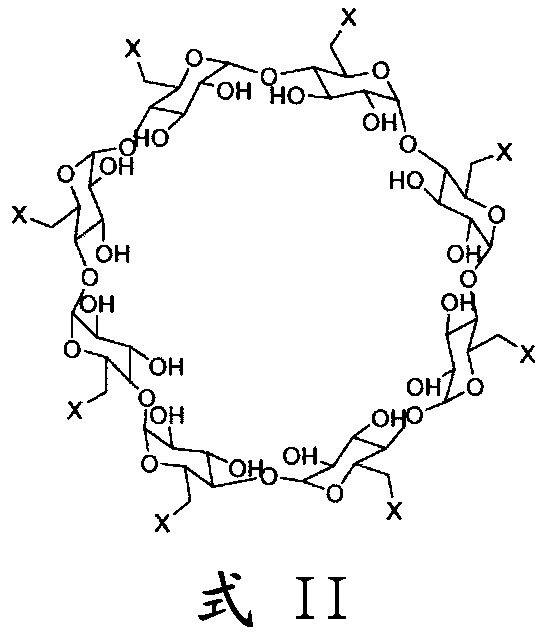
An improved process for the preparation of sugammadex
一种葡萄糖、巯基丙酸的技术,应用在改进的制备舒更葡萄糖领域,能够解决不可取、难工业规模中实施和控制、难以处理等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0115] In order to illustrate and explain the benefits of the present invention, an example of prior art has been carried out and presented as a reference example.
reference example 1
[0116] Reference Example 1 (Example 4 of WO2001 / 040316A1)
[0117] Preparation of sugammadex
[0118] Under nitrogen atmosphere, 3-mercaptopropionic acid (1.95 mL, 10 equiv) was dissolved in anhydrous dimethylformamide (72 mL) at room temperature. To this solution was added sodium hydride (1.97 g, 22 equiv, 60% dispersion in mineral oil) in three portions, and the mixture was stirred for a further 30 minutes. To this mixture was then added dropwise a solution of 6-per-deoxy-6-per-iodo-γ-cyclodextrin (5 g, 2.24 mmol) in anhydrous dimethylformamide (72 mL). After the addition, the reaction mixture was heated at 70 °C for 12 hours. After cooling, water (16 mL) was added to the mixture, and the volume was concentrated in vacuo to 64 mL. Ethanol (400 mL) was added, causing precipitation. The solid precipitate was collected by filtration and dialyzed for 36 hours. The volume was then reduced to 32 mL in vacuo. Ethanol was added thereto, and the precipitate was collected by fil...
reference example 2
[0119] Reference Example 2 (Example 2 of WO2012 / 025937A1)
[0120] Preparation of sugammadex
[0121] To a mixture of sodium hydride (60% dispersion in mineral oil, 3.13 g) in dimethylformamide (19 mL, 3.8 volumes) was slowly added 3-mercaptopropionic acid (3 mL, 10 equiv) under nitrogen. Solution in dimethylformamide (6 mL, 1.2 vol), keeping the temperature below 10 °C. The resulting mixture was stirred at 20-25°C for 30 minutes. 6-per-deoxy-6-per-chloro-γ-cyclodextrin (5 g) in dimethylformamide (50 mL, 10 volumes) was then slowly added under nitrogen at 5-10 °C, and The resulting mixture was heated to 70-75°C for 12 hours. The reaction mixture was cooled to 20-25 °C and dimethylformamide was partially removed under vacuum, and the reaction mixture was diluted with ethanol (75 mL, 15 vol). The resulting precipitate was stirred at 20-25°C for 1 hour and filtered, washed with ethanol (10 mL, 2 volumes) and dried under vacuum at 60-65°C. Obtained 9.4 g of an ivory colored s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com