Bioluminescent detection probe based on transcriptional activator like effector factor and construction method and application thereof
A technology of transcription activation and effector, applied in the field of biomedical analysis, can solve the problems of cumbersome operation, decreased luciferase stability and optical performance, and high price, and achieve the effect of high-sensitivity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
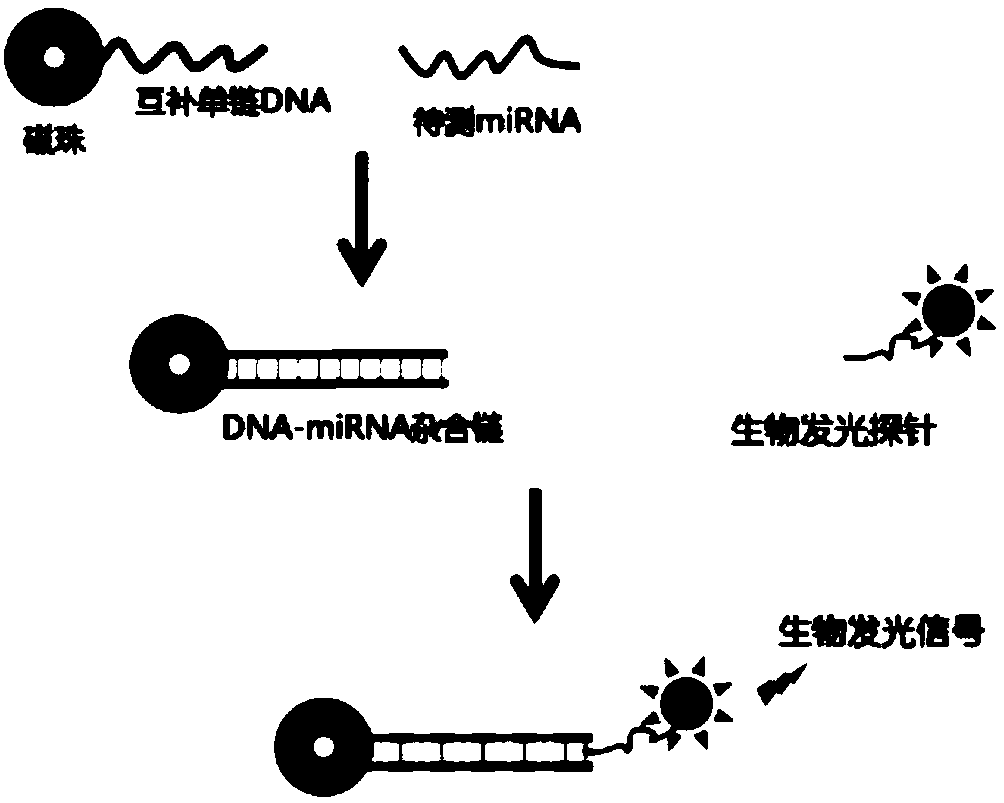
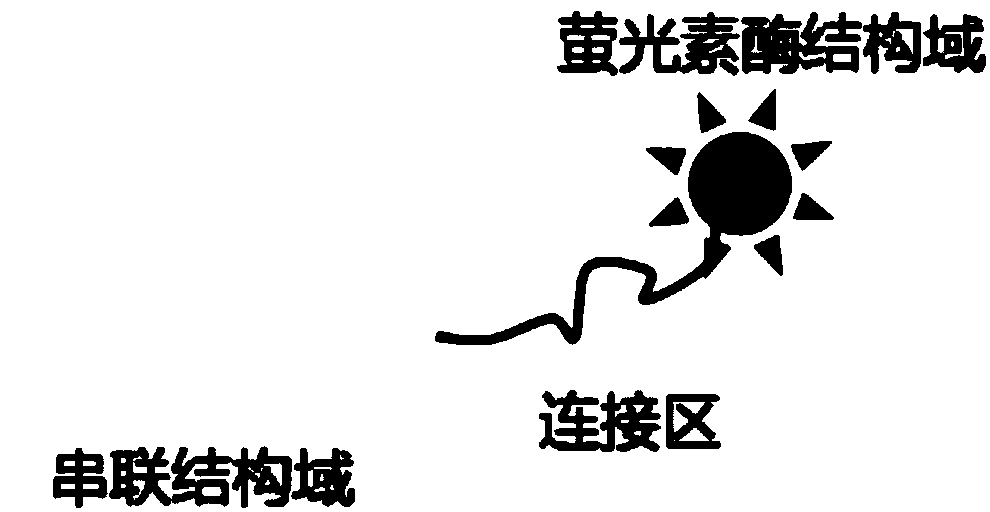
[0028] Example 1: In this example, the miR-21 sequence is used as the object to be detected, and the detection principle is as follows figure 1 shown. In this example, a bioluminescent probe composed of a transcriptional activator-like effector and a luciferase domain is first constructed, and the method is as follows:
[0029] (1) Design and synthesis of the coding fragments of each functional element of the probe: According to the codon preference of E. The coding fragment of the tandem domain and the coding sequence of the luciferase domain; the above coding fragment is prepared by the whole gene synthesis technology, and inserted into the pUC57 plasmid vector.
[0030] (2) Amplification and fusion of the coding fragments of each functional element of the probe: amplify the N-terminal and C-terminal domain coding fragments of transcriptional activator-like effectors by primers P1 and P2, and amplify the fragments at both ends of the amplified product Add the NdeI restrict...
Embodiment 2
[0036] Example 2: Application of a microRNA bioluminescence detection technology based on transcriptional activator-like effectors
[0037] (1) Preparation of ssDNA-coupled magnetic beads: 10 μM biotinylated ssDNA and 1 mg streptavidin-modified magnetic beads were mixed in 50 μL of 1×binding buffer (10 mM Tris-Cl, 1 mM EDTA , 2M NaCl, pH=7.5) for 4h; use a magnetic stand to separate the above magnetic beads, and use 200μL of 1×TALE buffer (20mM Tris-Cl, 60mM KCl, 5mM MgCl 2 , 2mM EDTA, 5% (v / v) glycerol, pH = 8.8) wash the magnetic beads 3 times, resuspend the magnetic beads with 100 μL 5% (w / v) BSA, and place them in a 4°C refrigerator overnight; The blocked magnetic beads were separated with a magnetic stand, resuspended with 100 μL of 1×TALEbuffer, and stored in a 4°C refrigerator until use. The biotinylated single-stranded DNA is complementary to miR-21, and the end is labeled with biotin, and its nucleotide sequence is shown in SEQ ID NO.33; the particles of the magnetic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com