Fuel cell catalyst carrier and preparation method thereof as well as cell electrodes
A catalyst carrier and fuel cell technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as poor electronic conductivity, achieve the effect of enhancing stability and reducing electrochemical corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
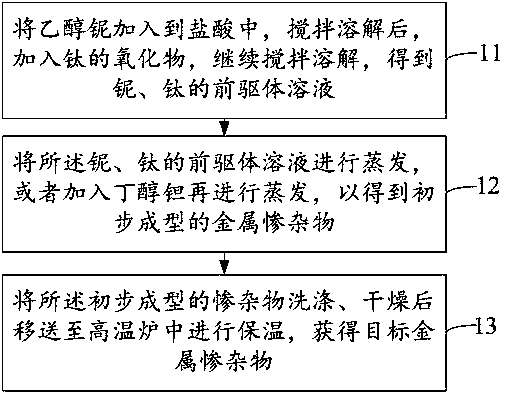
[0035] Embodiments of the present invention also provide a figure 1 The preparation method of the shown fuel cell catalyst carrier, it specifically comprises the following steps:
[0036] Step 11, adding niobium ethoxide into hydrochloric acid, stirring and dissolving, adding titanium oxide, continuing stirring and dissolving to obtain a niobium and titanium precursor solution.
[0037] Step 12, evaporating the precursor solution of niobium and titanium, or adding tantalum butoxide and evaporating, so as to obtain the initially shaped metal impurities.
[0038] Wherein, directly evaporating the precursor solution of niobium and titanium can obtain initially shaped but technical impurities, and the doping element of the single metal impurities is Nb; after adding tantalum butoxide, evaporate , then a preliminarily formed bimetallic dopant is obtained, and the doping elements of the bimetallic dopant are Ta and Nb.
[0039] Step 13, washing and drying the preliminarily formed ...
Embodiment 1-3
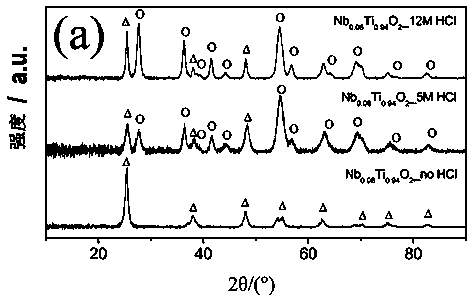
[0047] 1. Add 1.05mmol niobium ethoxide (Nb(OC 2 h 5 ) 5 ) into different concentrations of hydrochloric acid (concentration of hydrochloric acid corresponding to embodiment 1 is 0M (mole) / L, concentration of hydrochloric acid corresponding to embodiment 2 is 5M / L, concentration of hydrochloric acid corresponding to embodiment 3 is 12M / L), stirring and dissolving After 15 minutes, 15.30 mmol of isobutoxytitanium (Ti(OCH 2 CH 2 CH 2 CH 3 ) 4 ), continue stirring for 15 minutes, and dissolve to obtain a precursor solution of niobium and titanium.
[0048] 2. Add 244.6mmol of deionized water dropwise to the precursor solution of niobium and titanium, raise the temperature to 120°C, and keep it warm for 20 hours until all the solution evaporates to obtain the preliminary formed monometallic Nb miscellaneous matter.
[0049] 3. After washing the above-mentioned monometallic Nb impurities with deionized water, dry them overnight at 80° C., and finally, transfer the dried prod...
Embodiment 4
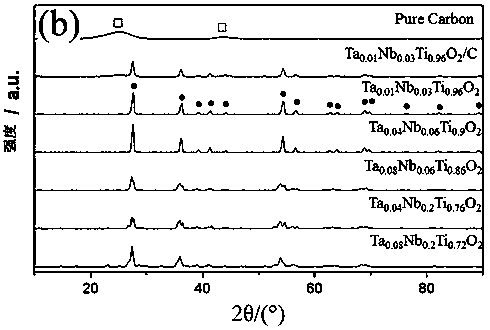
[0052] 1. Add 261mol niobium ethanol (Nb(OC 2 h 5 ) 5 ) into 965mmol hydrochloric acid (hydrochloric acid concentration is 12M / L), stirred and dissolved for 15 minutes, then added 7.60mmol titanium isopropoxide (Ti(OCH(CH 3 ) 2 ) 4 ), continue stirring for 15 minutes to form a precursor solution of niobium and titanium.
[0053] 2. Add 0.92mmol tantalum butoxide (Ta(OCH 2 CH 2 CH 2 CH 3 ) 5 ), after the mixed solution continued to stir for 15 minutes, it was heated to 120° C. and kept for 20 hours until all the solution evaporated to obtain preliminary formed bimetallic Nb and Ta impurities.
[0054] 3. Transfer the above-mentioned preliminary formed bimetallic Nb and Ta impurities to a high-temperature furnace, and obtain Ta 0.01 Nb 0.03 Ti 0.96 o 2 Bimetallic dopant.
[0055] 4, the Nb that embodiment 4 obtains 0.06 Ti 0.94 o 2 The phase structure of the metal dopant was determined and characterized respectively, and the XRD results are shown in Fig. 2(b). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com