Preparation method and application of fluorescent polymer
A technology of fluorescent polymers and fluorescent monomers, which is applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of few reports and how to regulate the performance of fluorescent polymers, and achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035]
[0036] The preparation method of the fluorescent polymer of the present invention includes (1) a step of synthesizing a fluorescent monomer and (2) a step of living radical polymerization, which will be described in detail below.
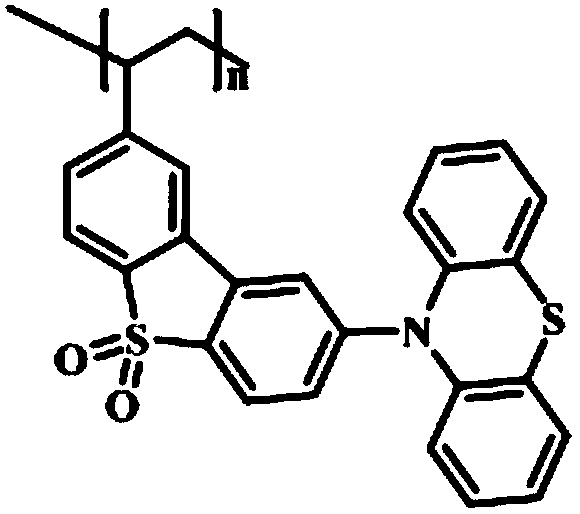
[0037] In the fluorescent monomer synthesis step (1), dibenzothiophene is used as a raw material to synthesize 3-vinyl-6-phenothiazine dibenzothiophene sulfone; it includes the synthesis process of 3,6-dibromodibenzothiophene, The synthesis process of 3,6-dibromodibenzothiophene sulfone, the synthesis process of 3-vinyl-6-bromodibenzothiophene sulfone and the synthesis process of 3-vinyl-6-phenothiazine dibenzothiophene sulfone.
[0038] During the synthesis of 3,6-dibromodibenzothiophene, at below 0°C, dibenzothiophene was added to chloroform, and then the chloroform solution containing liquid bromine was added dropwise, and the reaction was carried out at 20~30°C for 2~3 After the reaction was completed, 3,6-dibromodibenzothiophene was ...
Embodiment 1
[0069] (1) Synthesis of fluorescent monomer 3-vinyl-6-phenothiazine dibenzothiophene sulfone:
[0070] ①Synthesis of intermediate 3,6-dibromodibenzothiophene
[0071]
[0072] In an ice-water bath, 27.2 mmol of dibenzothiophene was added to a single-necked flask containing 30 ml of chloroform, and then a chloroform solution (obtained by dissolving 4 ml of liquid bromine in 5 ml of chloroform) was added dropwise, and reacted at 25°C for 2 days. After the reaction, 30 ml of saturated NaHSO was added 3 The organic layer was extracted with the aqueous solution. The organic layer was poured into a separatory funnel, extracted three times with dichloromethane, and washed three times with saturated aqueous NaCl solution until the aqueous layer became colorless. The organic layer was dried with anhydrous sodium sulfate and filtered, and the obtained filtrate was spin-dried in a rotary evaporator to obtain 8.54 g of a white solid with a yield of 92%.
[0073] ②Synthesis of 3,6-dib...
Embodiment 2
[0086] (1) Synthesis of fluorescent monomer 3-vinyl-6-phenothiazine dibenzothiophene sulfone:
[0087] It is the same as that of Embodiment 1 and will not be repeated here.
[0088] (2) Synthesis of fluorescent polymer PDPD-2
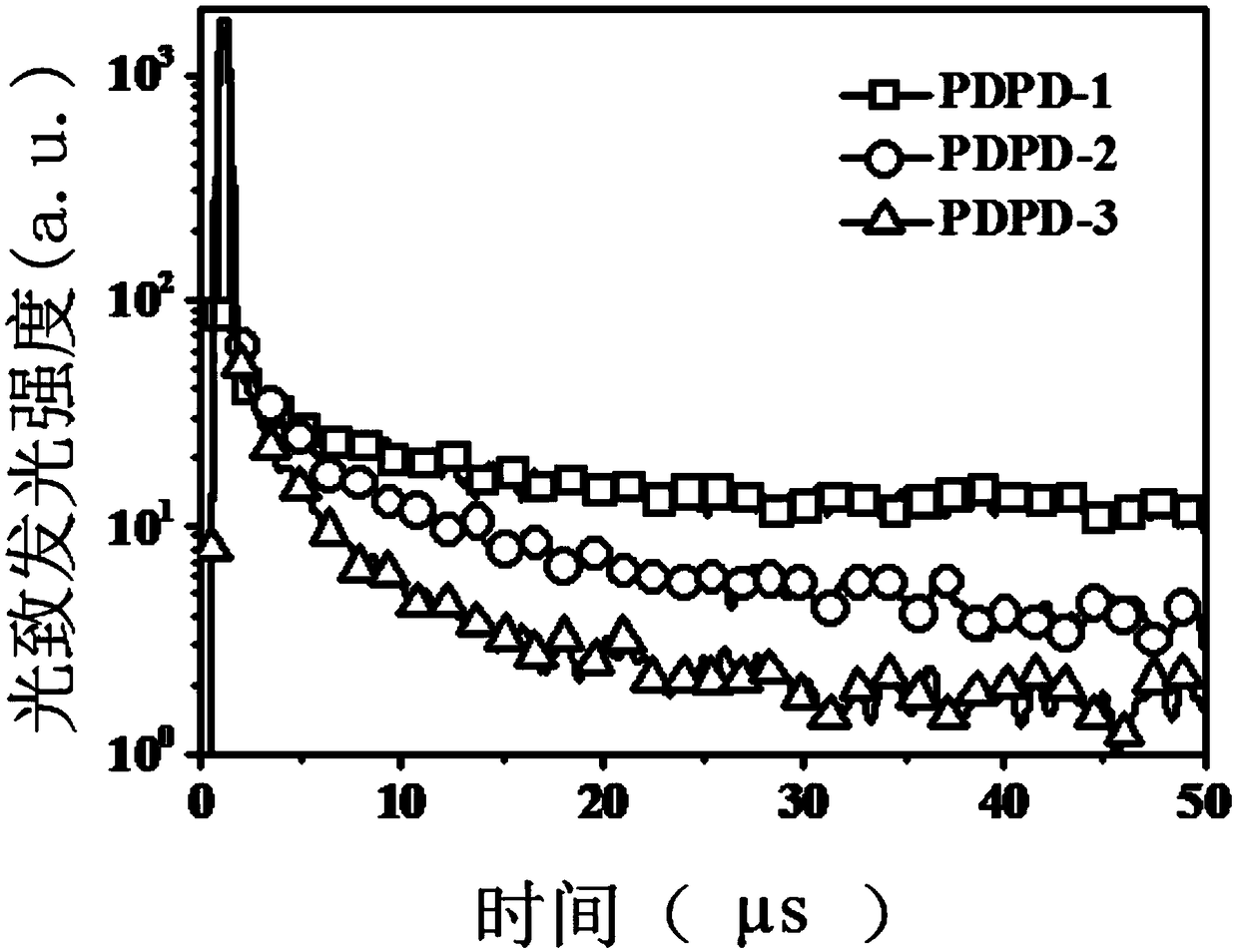
[0089] Add 0.03mmol of AIBN, 0.039mmol of TEMPO, 2mL of DMF and 3mmol of 3-vinyl-6-phenothiazine dibenzothiophene sulfone into a 5ml polymerization bottle, and seal it after freezing with liquid nitrogen three times and exhausting with argon Polymer bottle. Stir at 70°C for 24h, then raise the temperature to 135°C for 48h, quench to terminate the reaction; pour the reaction liquid into 20-30 times the volume of ethanol, filter, wash the filter cake with ethanol repeatedly for 3 times, filter, and vacuum-dry. The light yellow powder polymer PDPD-2 was obtained. Fluorescence performance test results see figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com