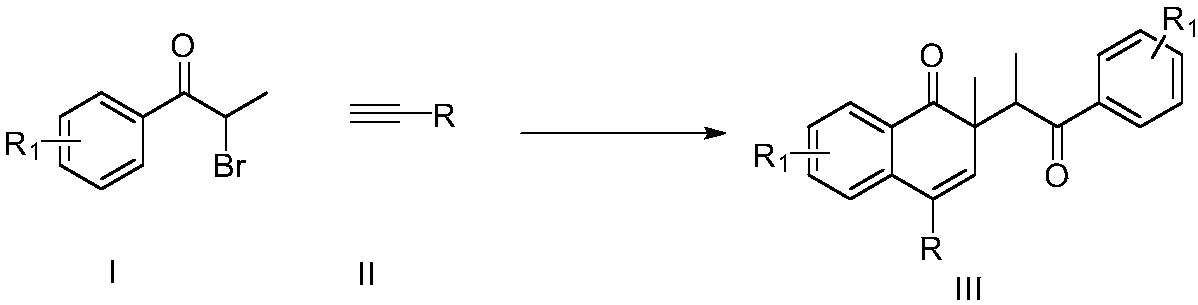
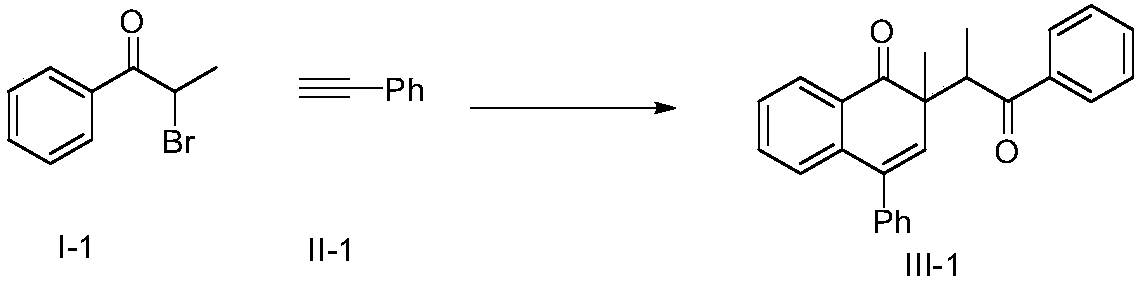
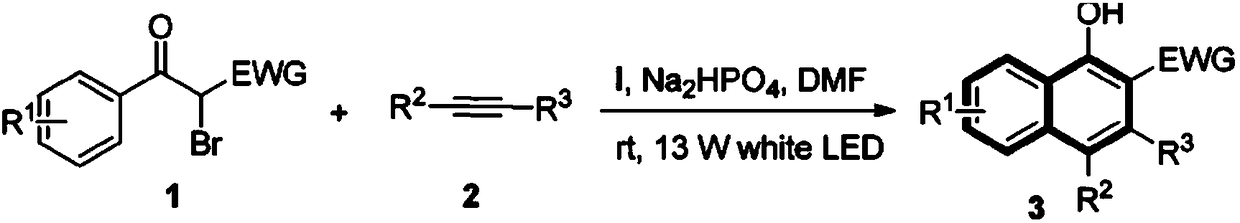Preparation method of naphthalenone compound
The technology of a compound and a synthetic method is applied in the field of preparation of naphthone compounds, and can solve the problems of environmental pollution, narrow substrate adaptation range, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 20
[0046]
[0047] Add 2-bromopropiophenone (0.2mmol) shown in the formula I-1 to the Schlenk lock reactor, p-methylphenylacetylene (0.1mmol) shown in the formula II-2, Cu(MeCN) 4 PF 6 (10mol%), 1,10-phen (20mol%), K 2 CO 3 (2equiv) and toluene (2mL), under argon atmosphere, the reaction was stirred at 120°C, and the reaction was detected by TLC (about 16 hours), and then the reaction solution was filtered through a short silica gel column, and ethyl acetate washed the filter cake, and the Concentrate under reduced pressure to obtain a residue, which is separated by silica gel column chromatography to obtain the target product of formula III-2. Yield 78%; d.r.=1:1; colorless oily liquid; 1 H NMR (400MHz, CDCl 3)δ:8.17(d,J=7.6Hz,0.5H),8.03(d,J=8.0Hz,1H),7.97(d,J=7.6Hz,0.5H),7.84(d,J=7.6Hz, 1H),7.59-7.36(m,5H),7.30-7.20(m,5H),6.51(s,0.5H),6.19(s,0.5H),4.41-4.29(m,1H),2.41(s, 3H), 1.39(s, 1.50H), 1.36(d, J=7.2Hz, 1.50H), 1.29(s, 1.50H), 1.14(d, J=6.8Hz, 1.50H).; 13 C NMR (...
Embodiment 21
[0049]
[0050] Add 2-bromopropiophenone (0.2mmol) shown in the formula I-1 to the Schlenk lock reactor, p-chlorophenylacetylene (0.1mmol) shown in the formula II-3, Cu(MeCN) 4 PF 6 (10mol%), 1,10-phen (20mol%), K 2 CO 3 (2equiv) and toluene (2mL), under argon atmosphere, the reaction was stirred at 120°C, and the reaction was detected by TLC (about 16 hours), and then the reaction solution was filtered through a short silica gel column, and ethyl acetate washed the filter cake, and the Concentrate under reduced pressure to obtain a residue, which is separated by silica gel column chromatography to obtain the target product of formula III-3. Yield 75%; d.r.=1.5:1; white solid; 1 H NMR (400MHz, CDCl 3 )δ: 8.19(d, J=7.6Hz, 0.41H), 8.04(d, J=7.6Hz, 0.82H), 7.98(d, J=7.6Hz, 0.66H), 7.85(d, J=7.6Hz ,1.28H),7.52-7.32(m,9H),7.14(t,J=7.6Hz,1H),6.54(s,0.41H),6.22(s,0.62H),4.41-4.33(m,1H) ,1.39(s,1.82H),1.37(d,J=6.8Hz,1.87H),1.30(s,1.21H),1.13(d,J=6.8Hz,1.23H); 13 C NMR (100MH...
Embodiment 22
[0052]
[0053] Add 2-bromopropiophenone (0.2mmol) shown in the formula I-1 to the Schlenk lock reactor, p-acetylphenylacetylene (0.1mmol) shown in the formula II-4, Cu(MeCN) 4 PF 6 (10mol%), 1,10-phen (20mol%), K 2 CO 3 (2equiv) and toluene (2mL), under argon atmosphere, the reaction was stirred at 120°C, and the reaction was detected by TLC (about 16 hours), and then the reaction solution was filtered through a short silica gel column, and ethyl acetate washed the filter cake, and the Concentrate under reduced pressure to obtain a residue, which is separated by silica gel column chromatography to obtain the target product of formula III-4. Yield 54%; d.r.>20:1; colorless oily liquid; 1 H NMR (400MHz, CDCl 3 )δ: 8.02(t, J=8.4Hz, 3H), 7.86(d, J=7.6Hz, 2H), 7.54-7.45(m, 4H), 7.41(t, J=7.6Hz, 2H), 7.34( t,J=7.6Hz,1H),7.13(d,J=8.0Hz,1H),6.27(s,1H),4.45-4.32(m,1H),2.66(s,3H),1.41(s,3H ), 1.38(d, J=6.8Hz, 3H); 13 C NMR (100MHz, CDCl 3 )δ: 202.8, 202.8, 197.7, 144.8, 137....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com