Methods of vitamin d treatment
A technology of hydroxyvitamins and hydroxyvitamins, which is applied in the field of treatment of vitamin D-responsive diseases, and can solve problems such as elevated PTH
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0091]Extended Release 25-Hydroxyvitamin D 3 Phase III clinical trial in patients with CKD stage 3 or 4
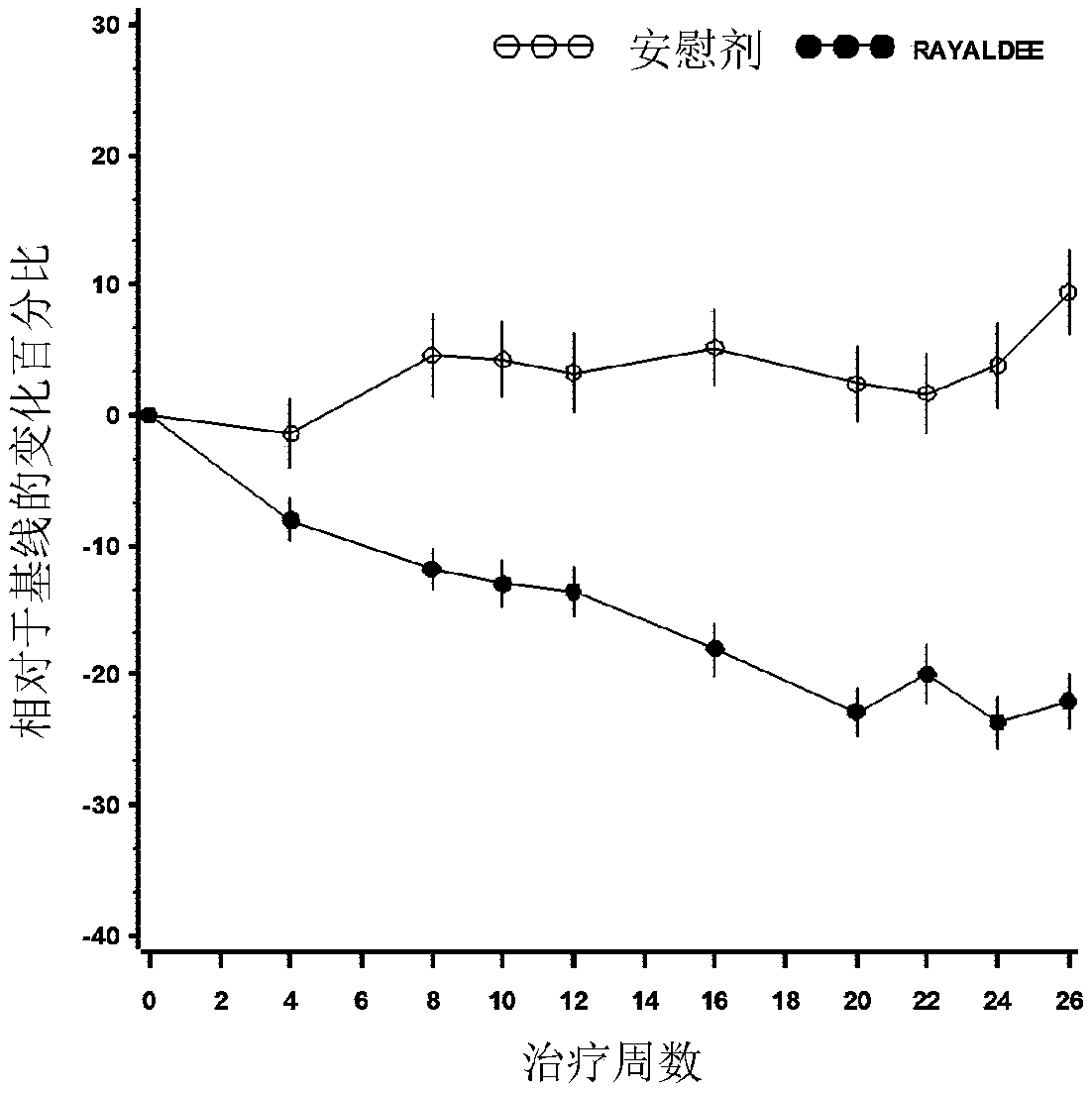
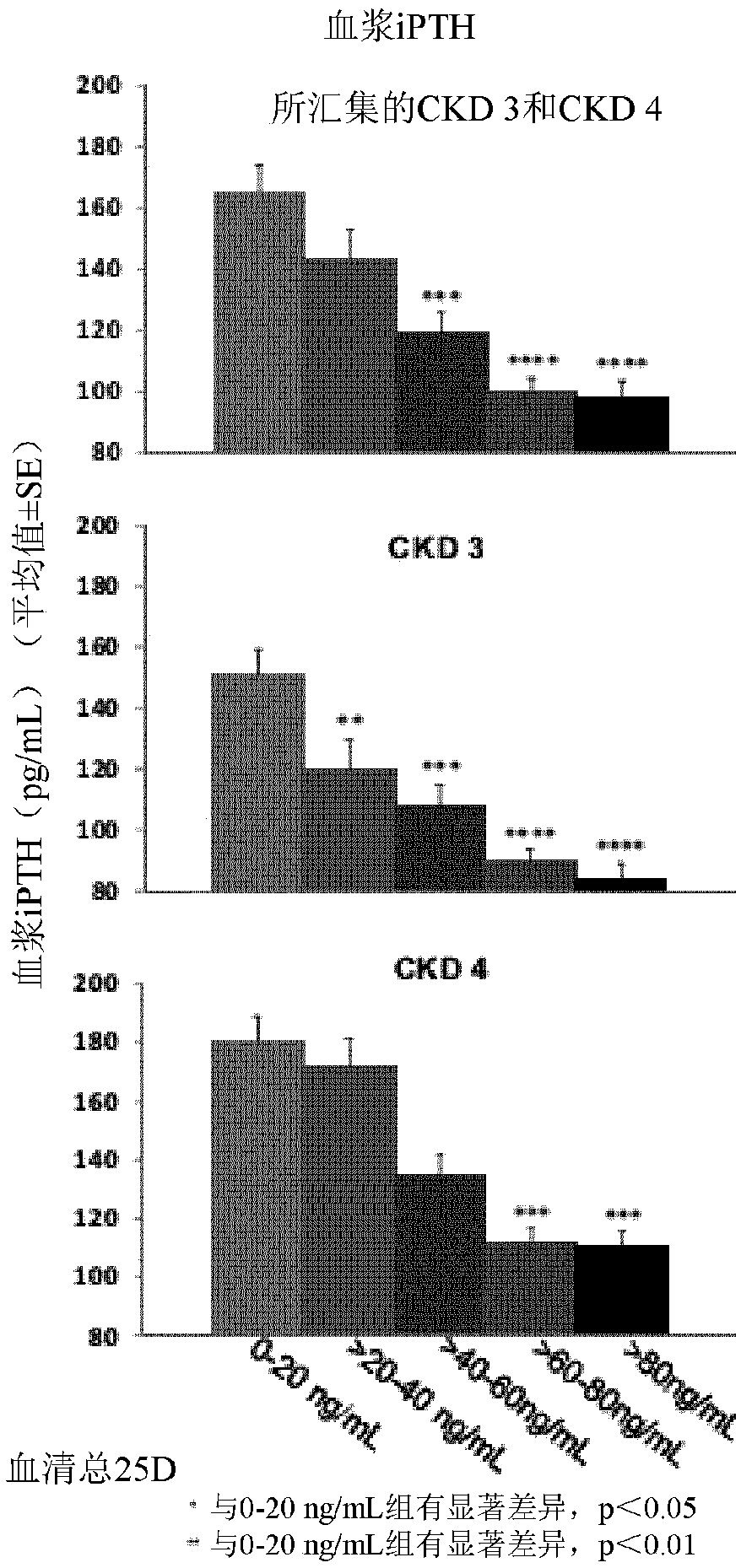
[0092] 25-Hydroxyvitamin D 3 The efficacy and safety of the extended-release formulation (RAYALDEE, Opko Ireland Global Holdings Ltd.) was evaluated in two identical multicenter, randomized, placebo-controlled, double-blind studies in patients with secondary parathyroid High energy syndrome (iPTH>85pg / mL), stage 3 or 4 chronic kidney disease and related serum total 25-hydroxyvitamin D content ≥10ng / ml and ≤30ng / ml. Subjects were stratified by disease stage and randomized in a 2:1 ratio to receive an oral dose of RAYALDEE (or matching placebo) 30 micrograms once daily at bedtime for 12 weeks, followed by 30 or 60 micrograms once daily at bedtime. Microgram oral doses of RAYALDEE (or placebo) were treated for an additional 14 weeks. Each capsule contains the following excipients: mineral oil, mono- and diglycerides, paraffin, hypromellose, lauroyl polyoxyglycerides, dehy...
example 2
[0104] Pharmacokinetic Studies
[0105]To evaluate the pharmacokinetics of RAYALDEE in healthy subjects and subjects with Stage 3 or 4 CKD. Following repeated daily doses of RAYALDEE at bedtime to subjects with secondary hyperparathyroidism, chronic kidney disease and vitamin D insufficiency, exposure to calcifediol increased proportionally over the dose range of 30 to 90 micrograms. After about 3 months, a plateau of serum total 25-hydroxyvitamin D is reached. Following multiple dose administration of RAYALDEE, mean steady-state concentrations of serum total 25-hydroxyvitamin D were 53 ng / mL and 68 ng / mL for the 30 mcg and 60 mcg dose groups, respectively. In terms of distribution, calcifediol is extensively bound (>98%) to plasma proteins. Following a single oral dose of RAYALDEE, the mean apparent volume of distribution was 8.8 L in healthy subjects and after repeated dosing it was 30.1 L in subjects with Stage 3 or 4 chronic kidney disease. With regard to elimination,...
example 3
[0113] Extended Release 25-Hydroxyvitamin D 3 Clinical trial in patients with stage 5 CKD
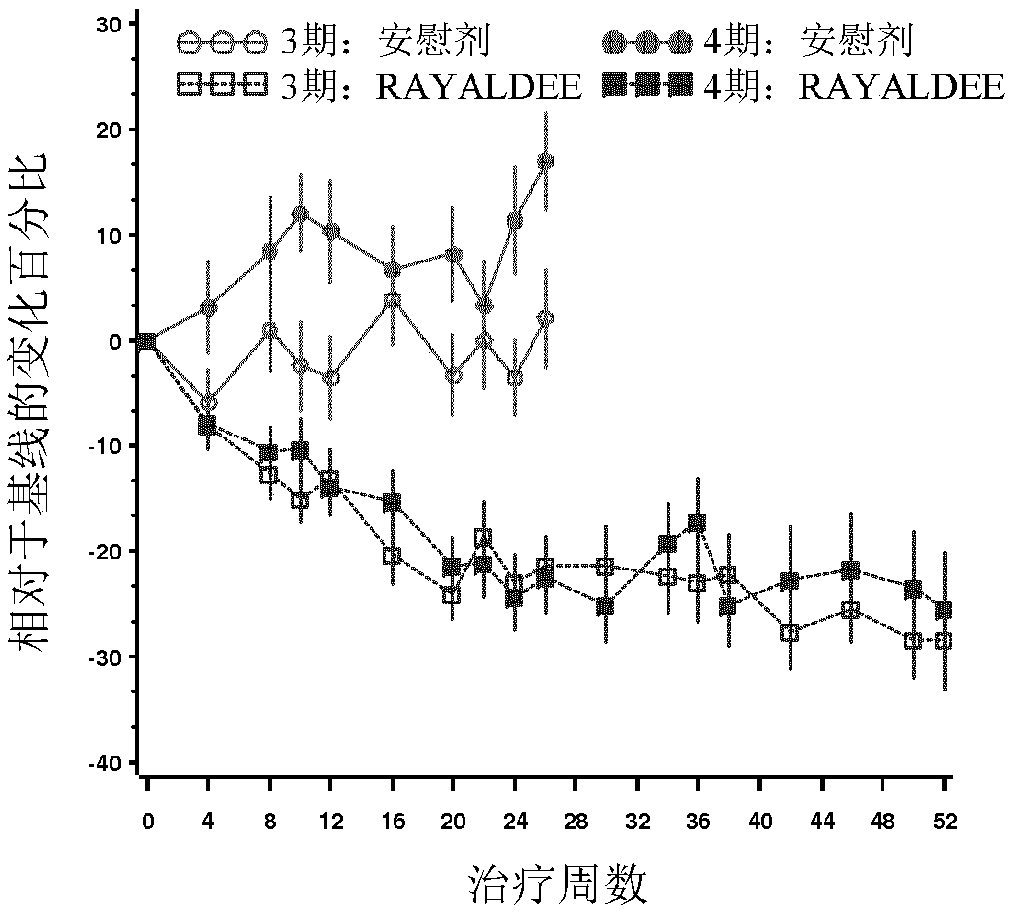
[0114] A multicentre, randomized, double-blind, placebo-controlled clinical study in subjects with secondary hyperparathyroidism, vitamin D insufficiency, and stage 5 CKD undergoing thrice-weekly hemodialysis to evaluate Three weekly doses of 25-hydroxyvitamin D 3 Safety and Efficacy of Extended Release Formulations in Reducing Serum Intact Parathyroid Parathyroid (iPTH) by At Least 30% from Pre-Treatment Baseline Relative to Placebo. 25-Hydroxyvitamin D 3 The extended-release formulation is provided in capsules that have the same formulation as RAYALDEE but contain 150 micrograms of 25-hydroxyvitamin D 3 . Approximately 600 subjects were screened to divide approximately 280 eligible subjects balanced in severity of SHPT into four parallel groups in a 1:1:1:1 ratio to receive the following regimens for 52 weeks: (a) 300 per week micrograms of 25-hydroxyvitamin D 3 extended-relea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com