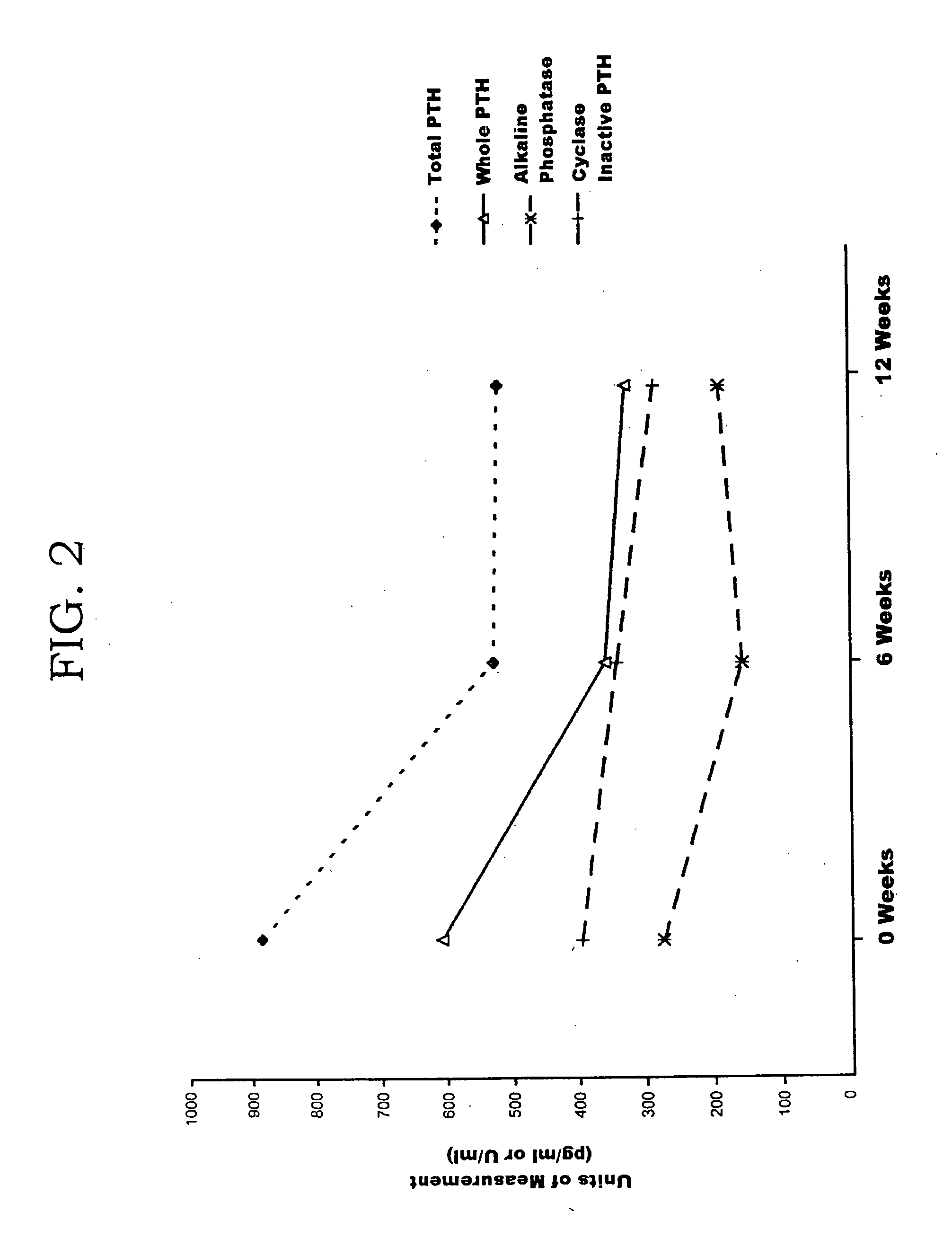
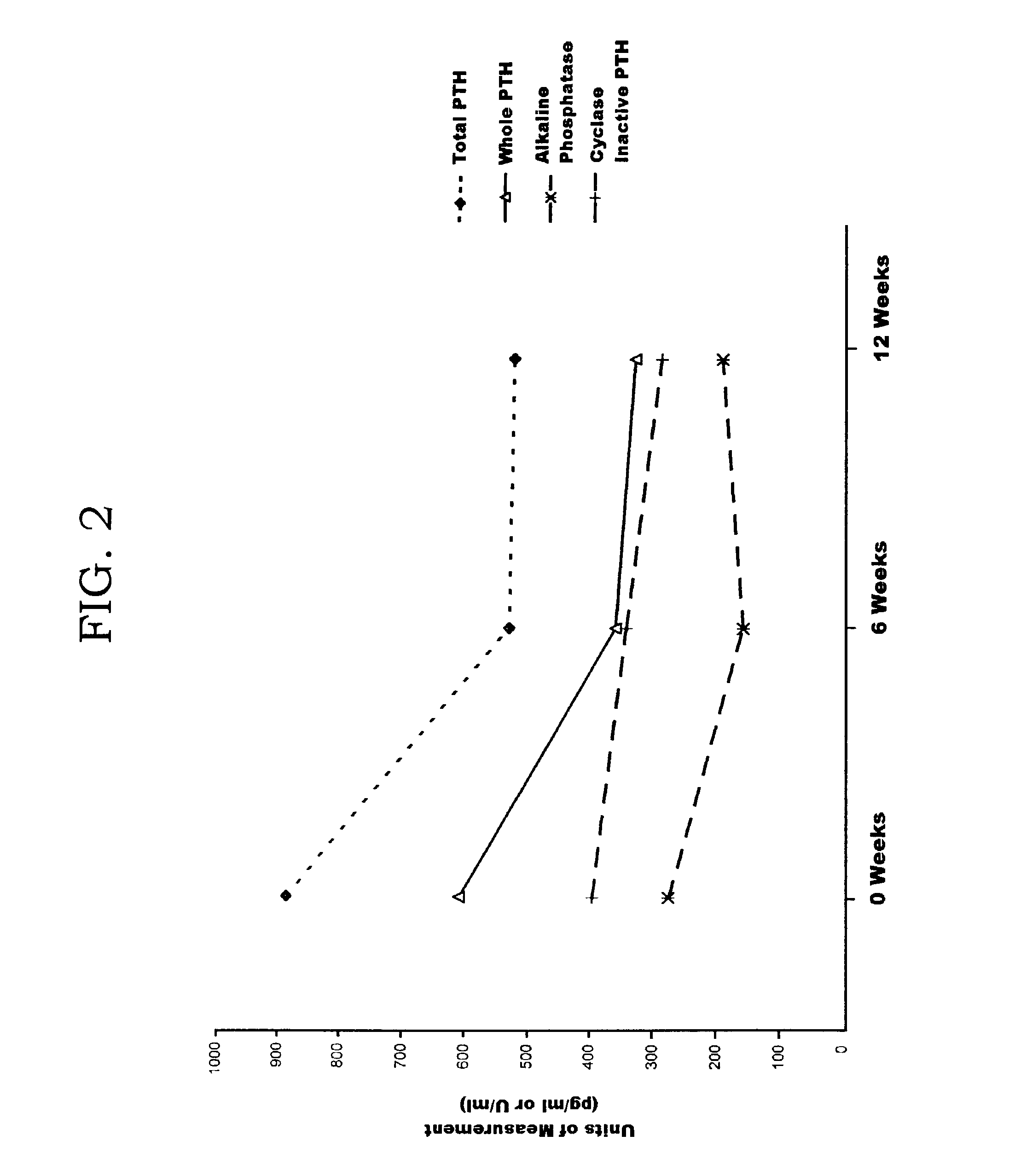
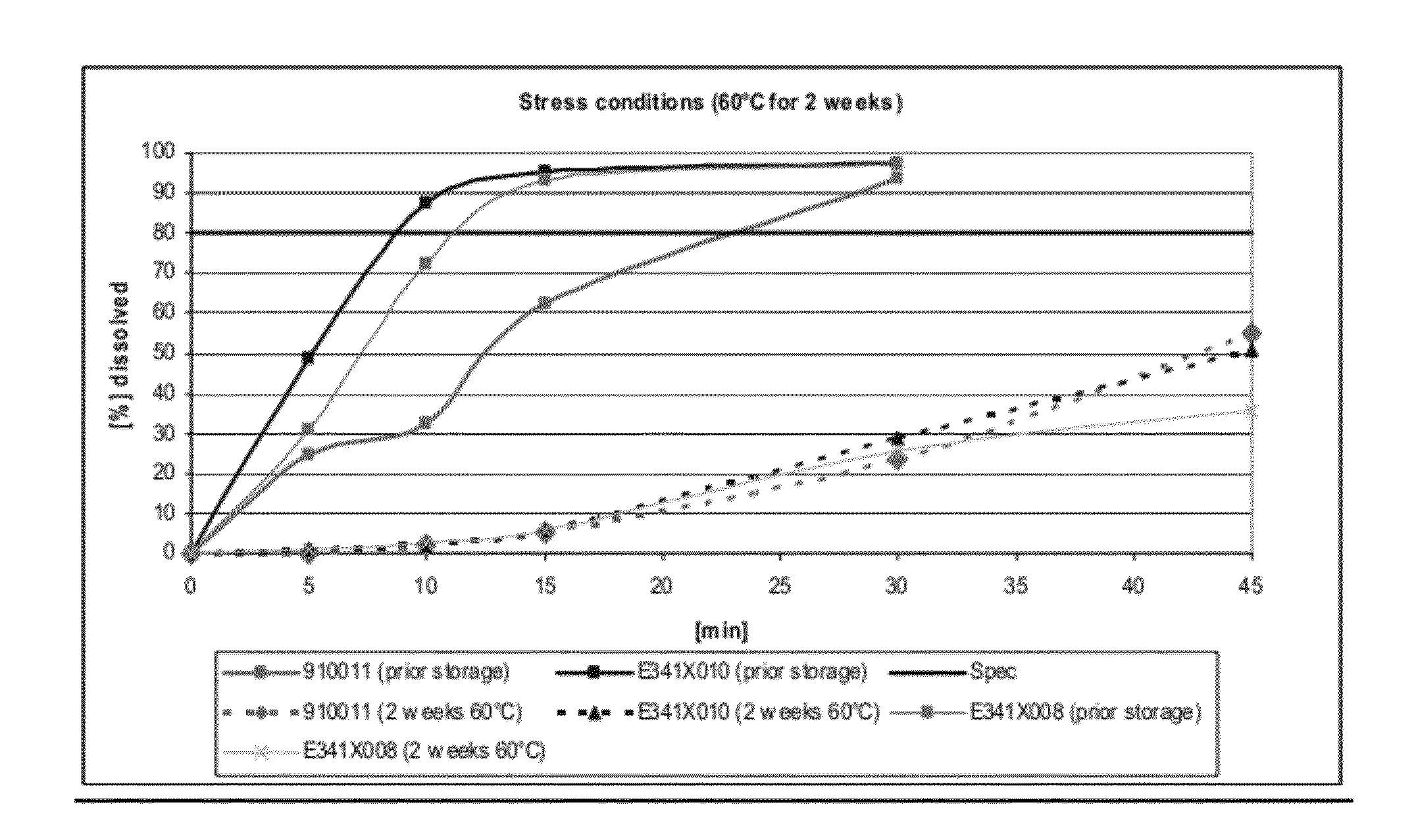
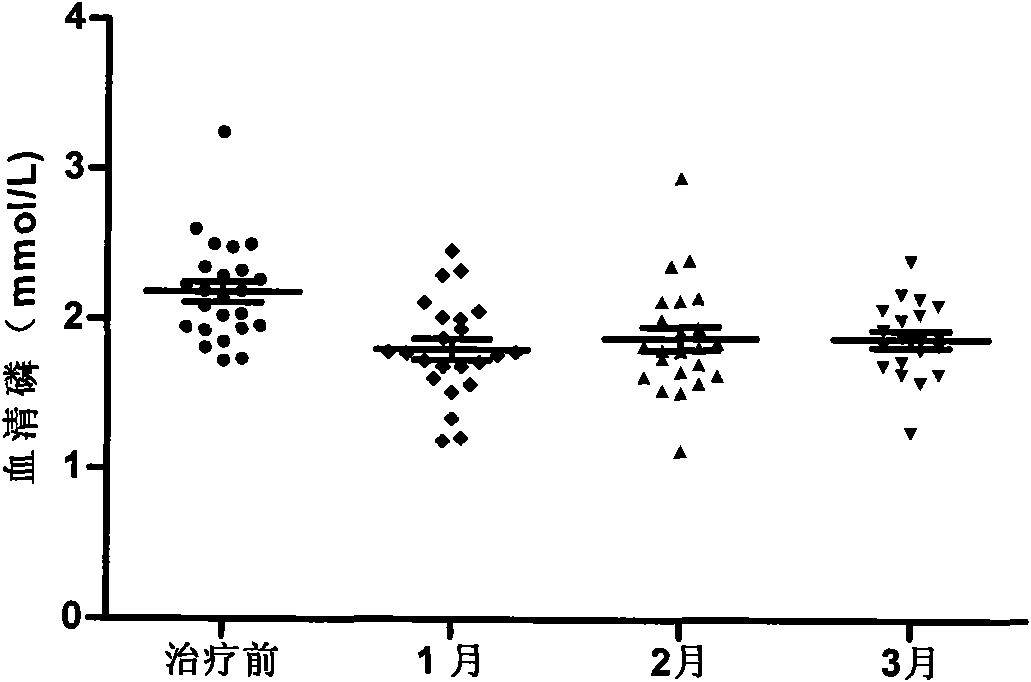
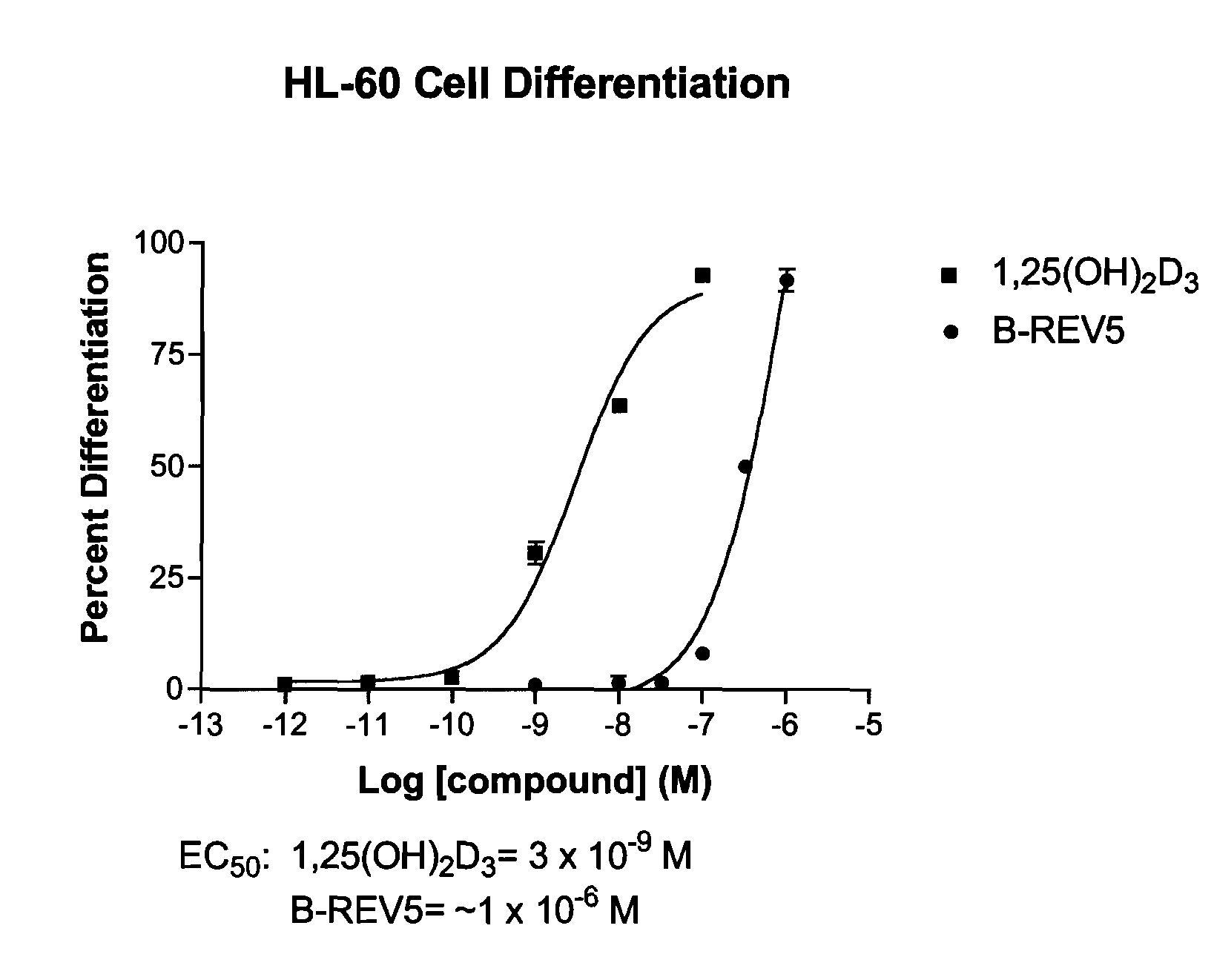
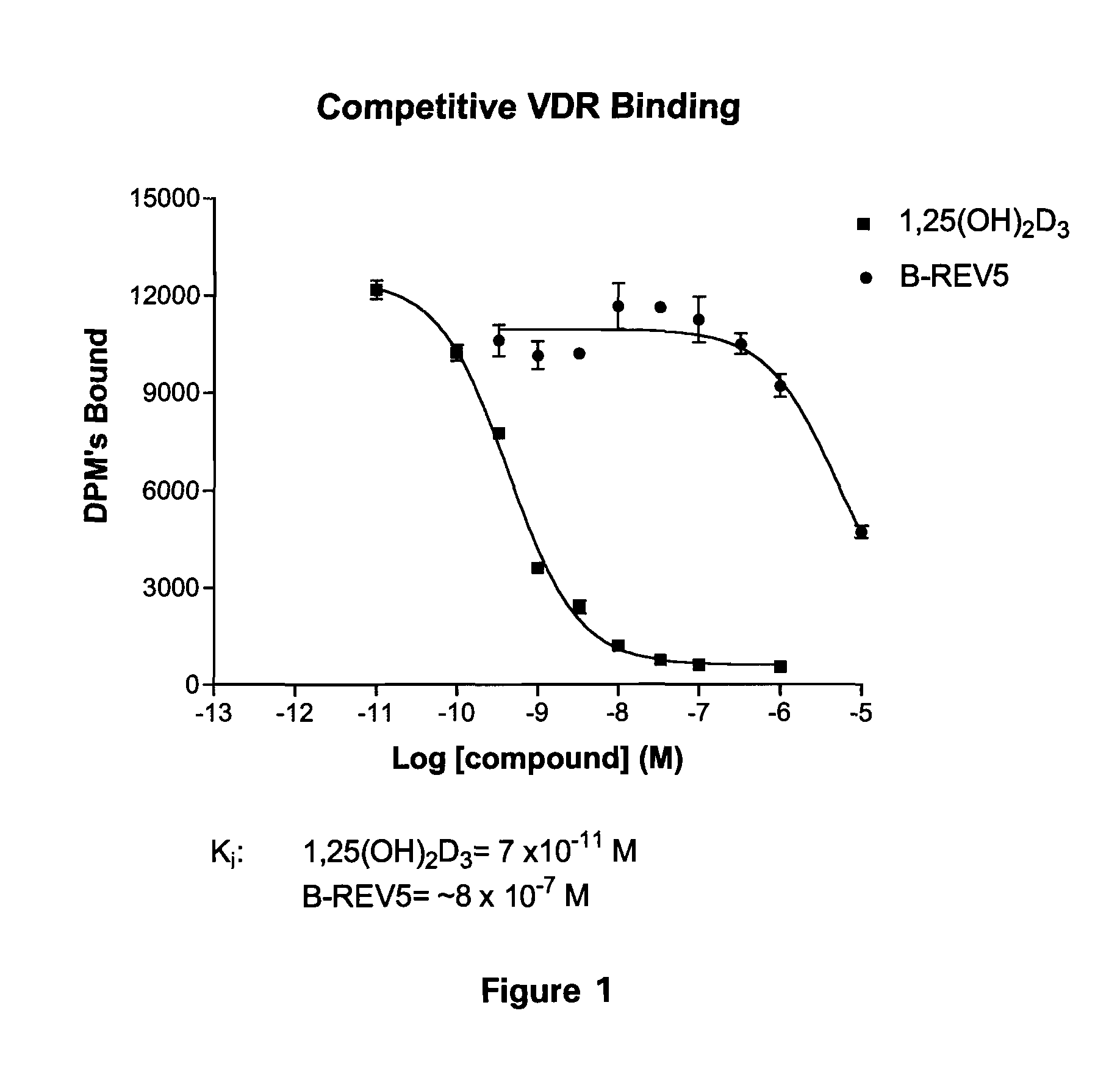
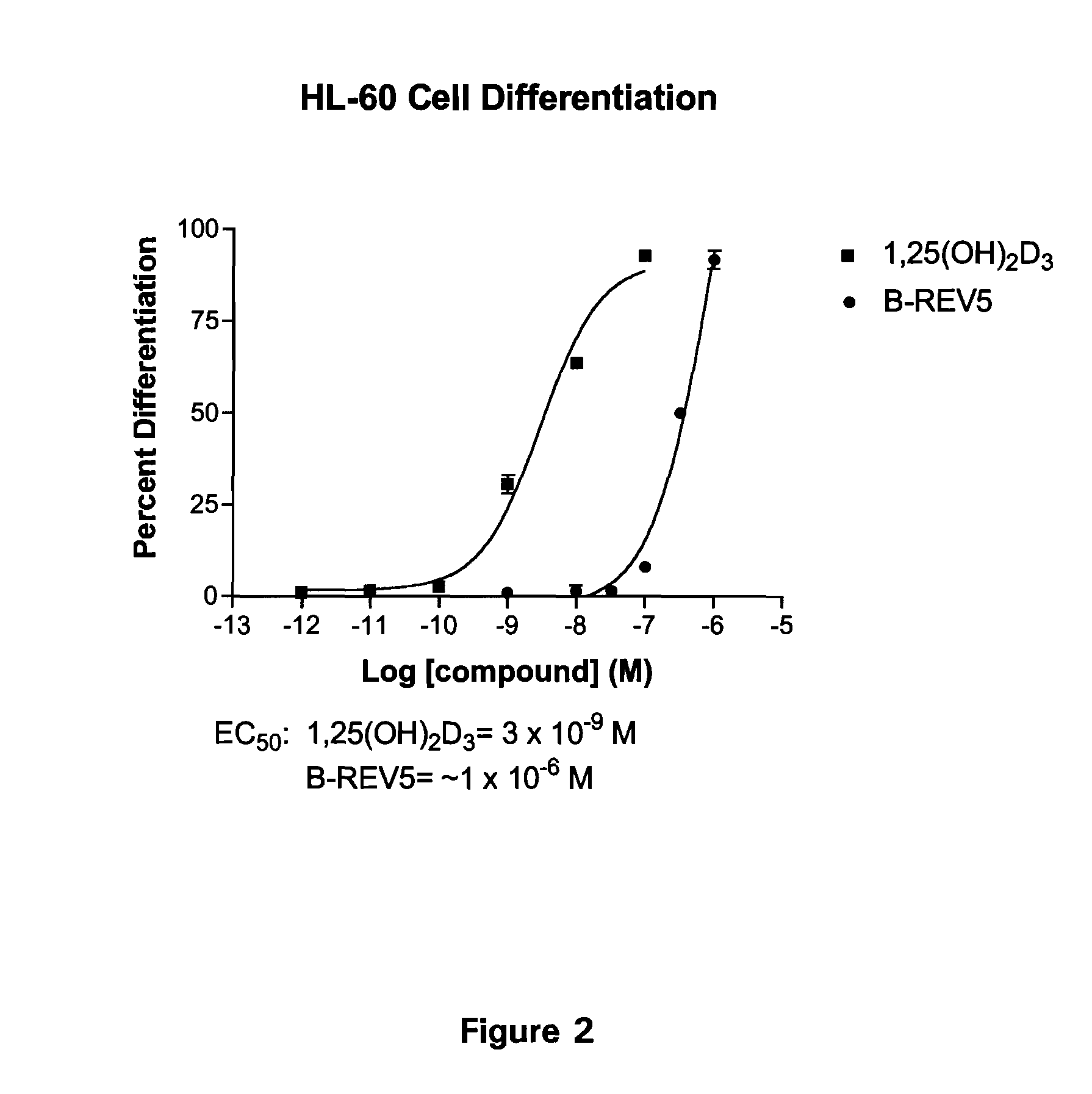
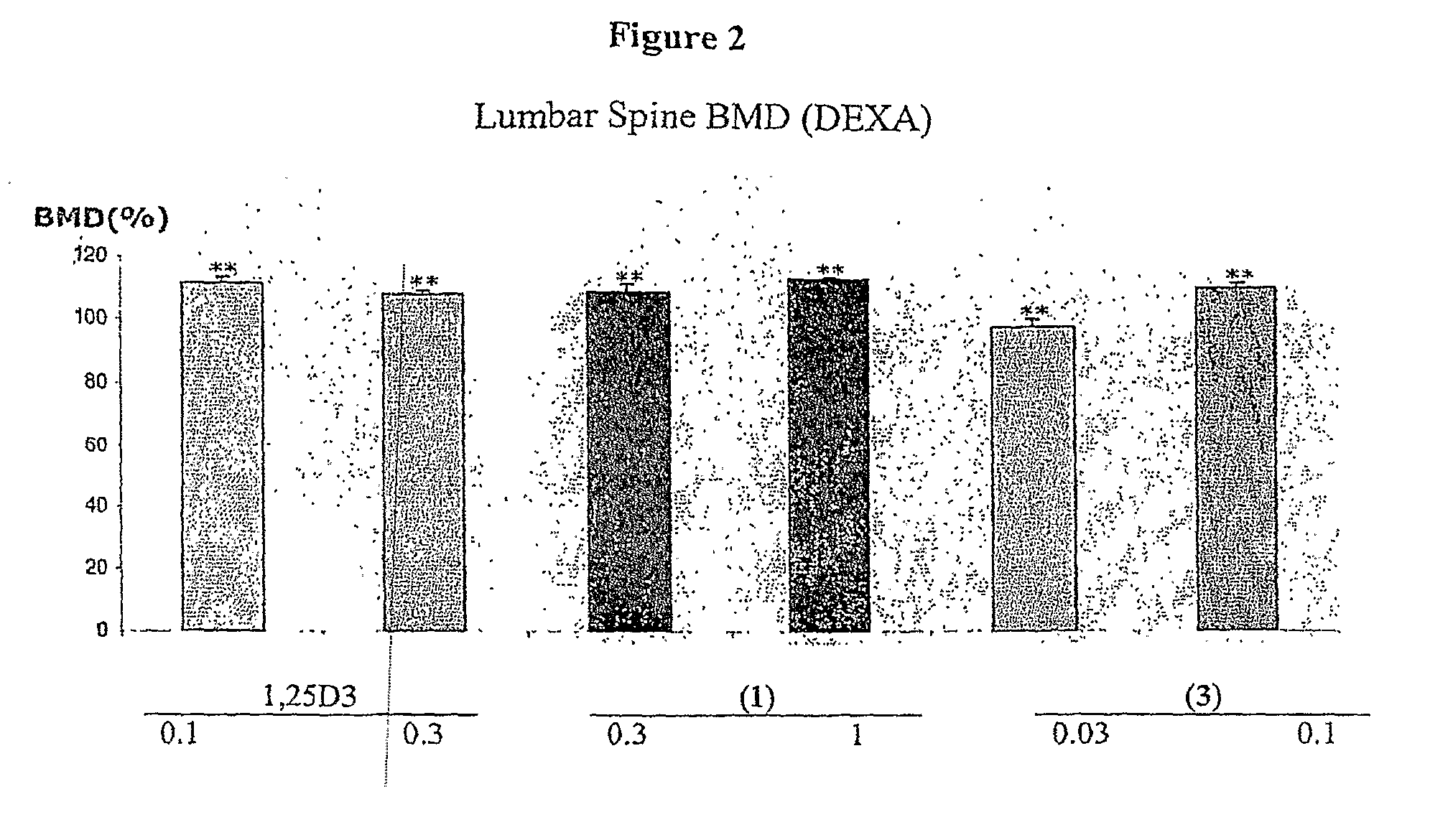
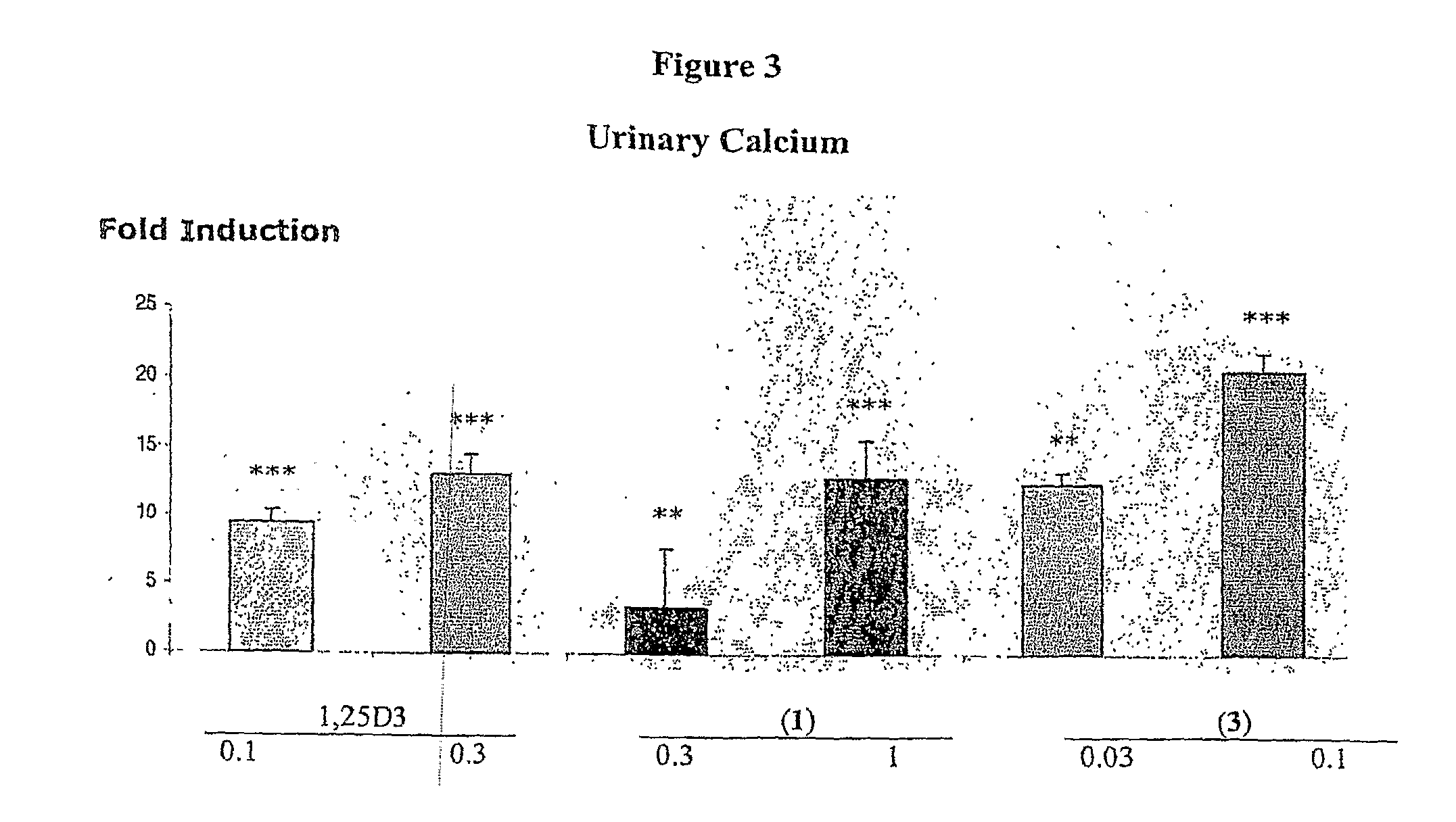
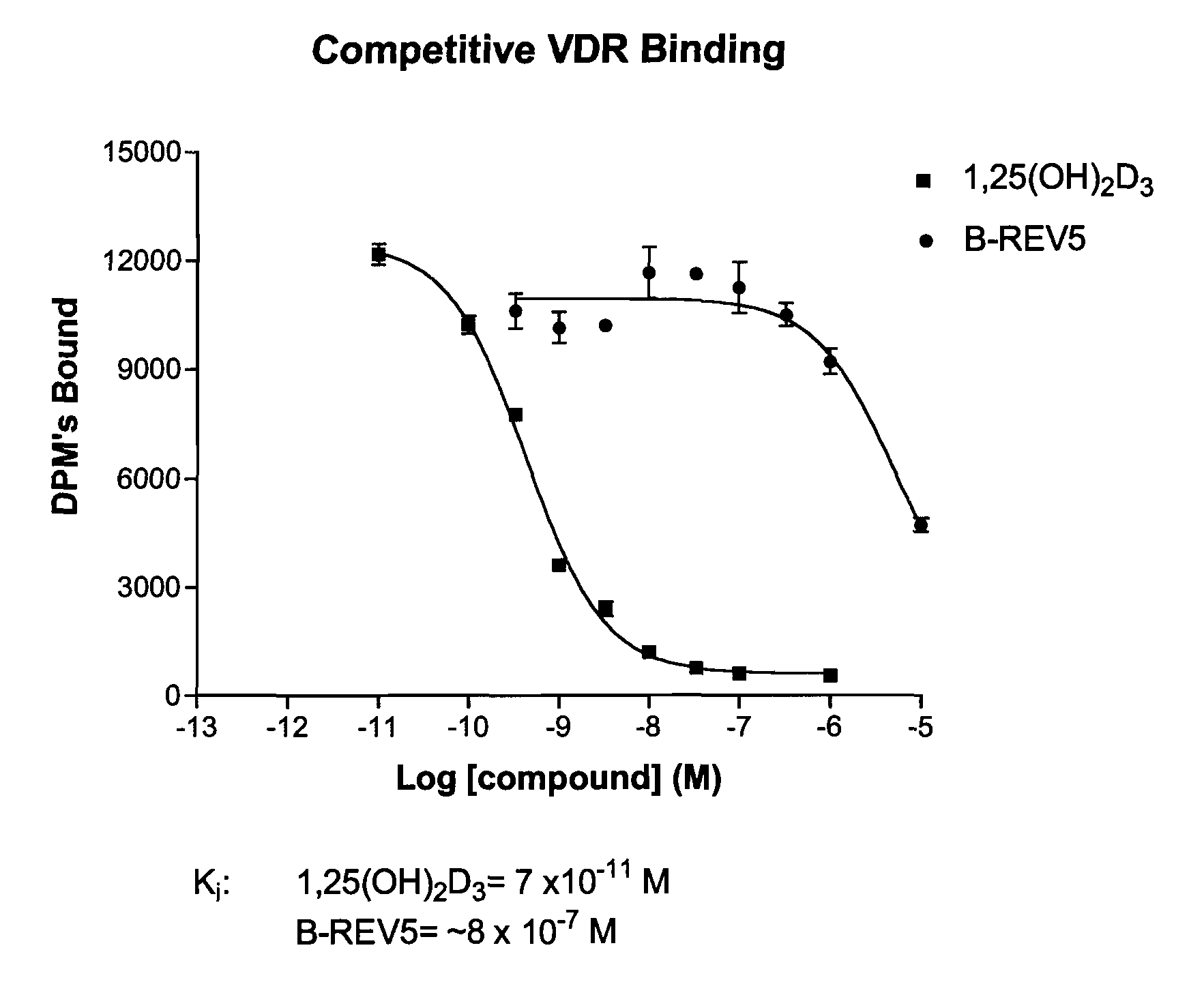
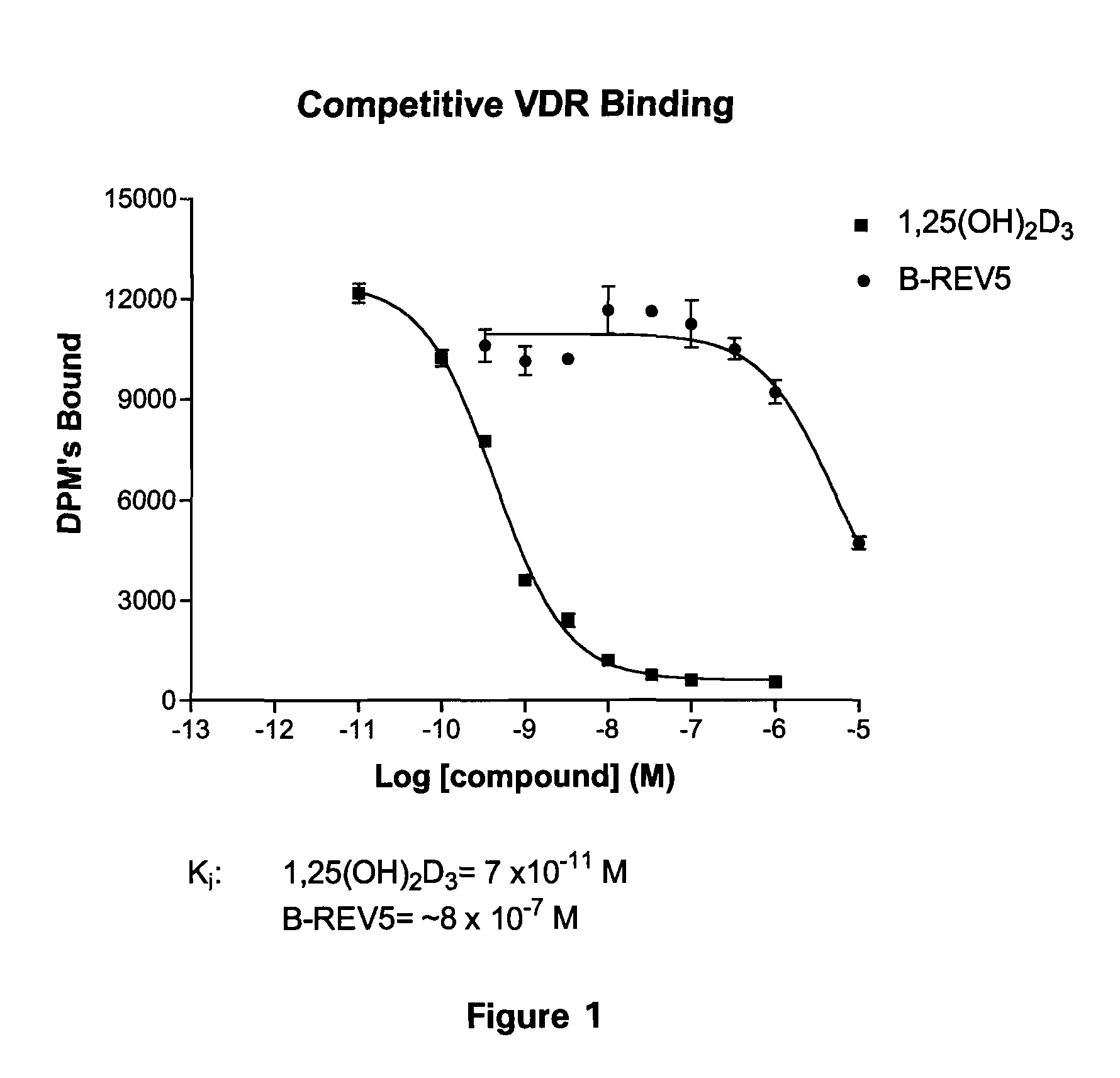
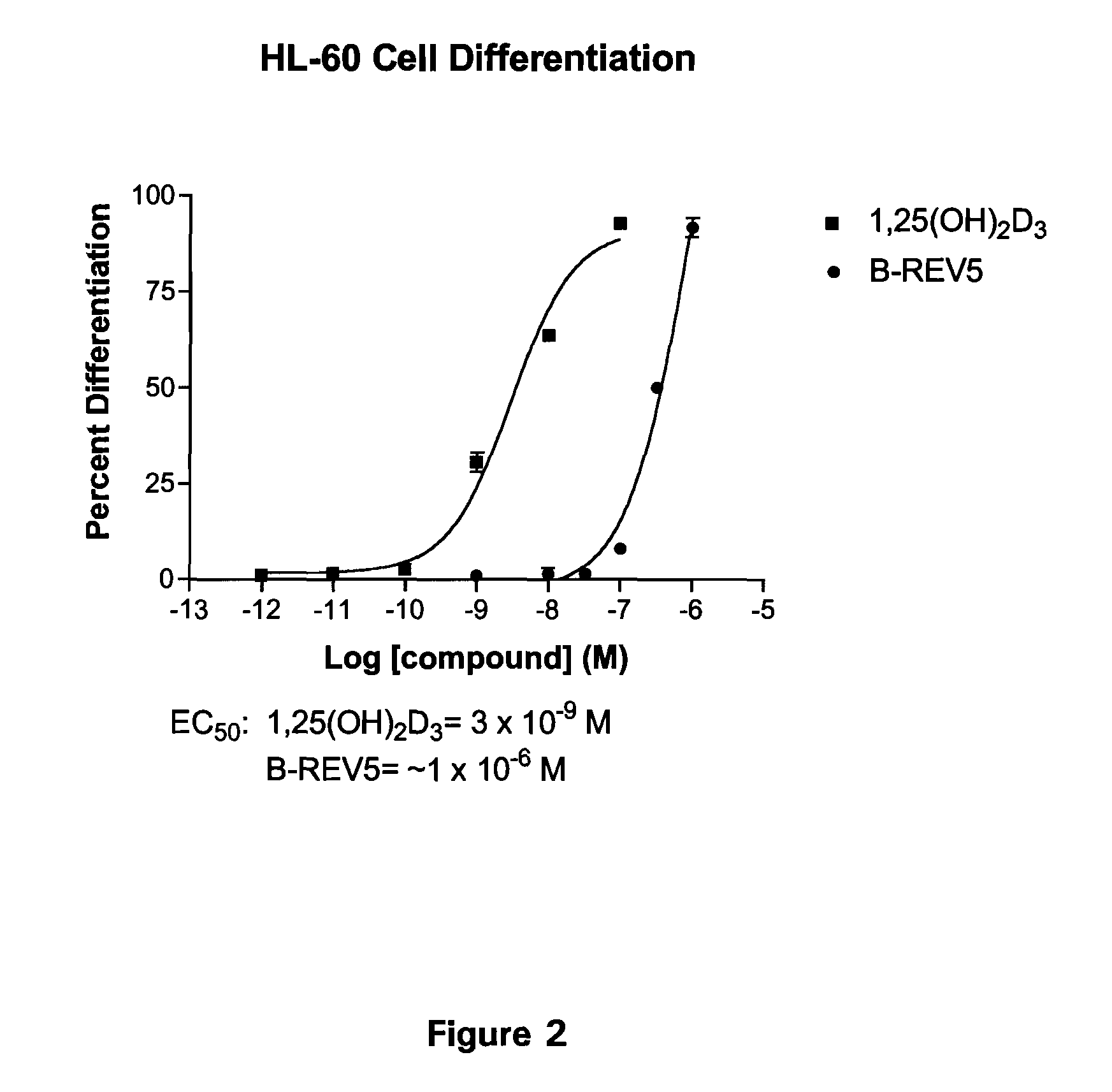
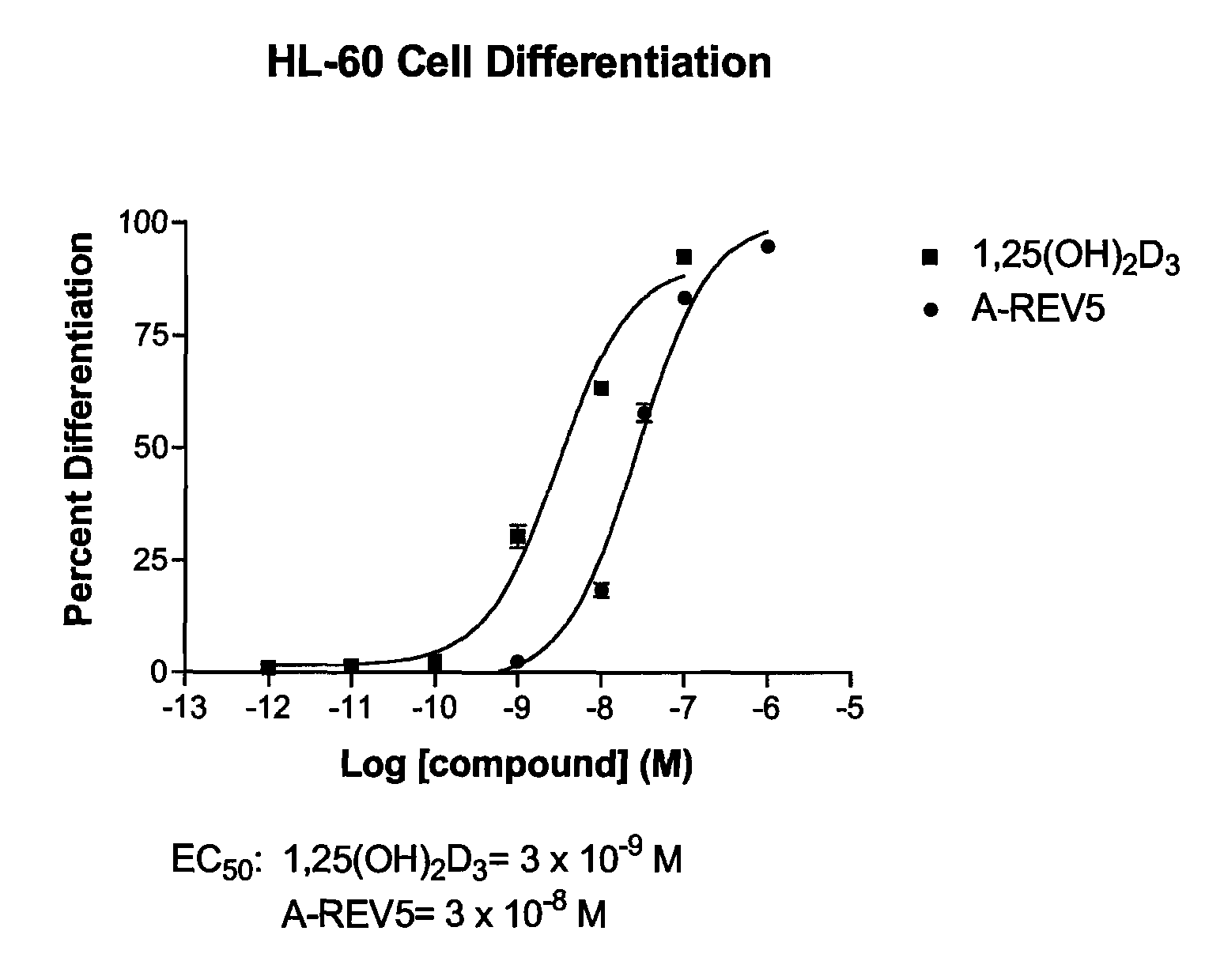
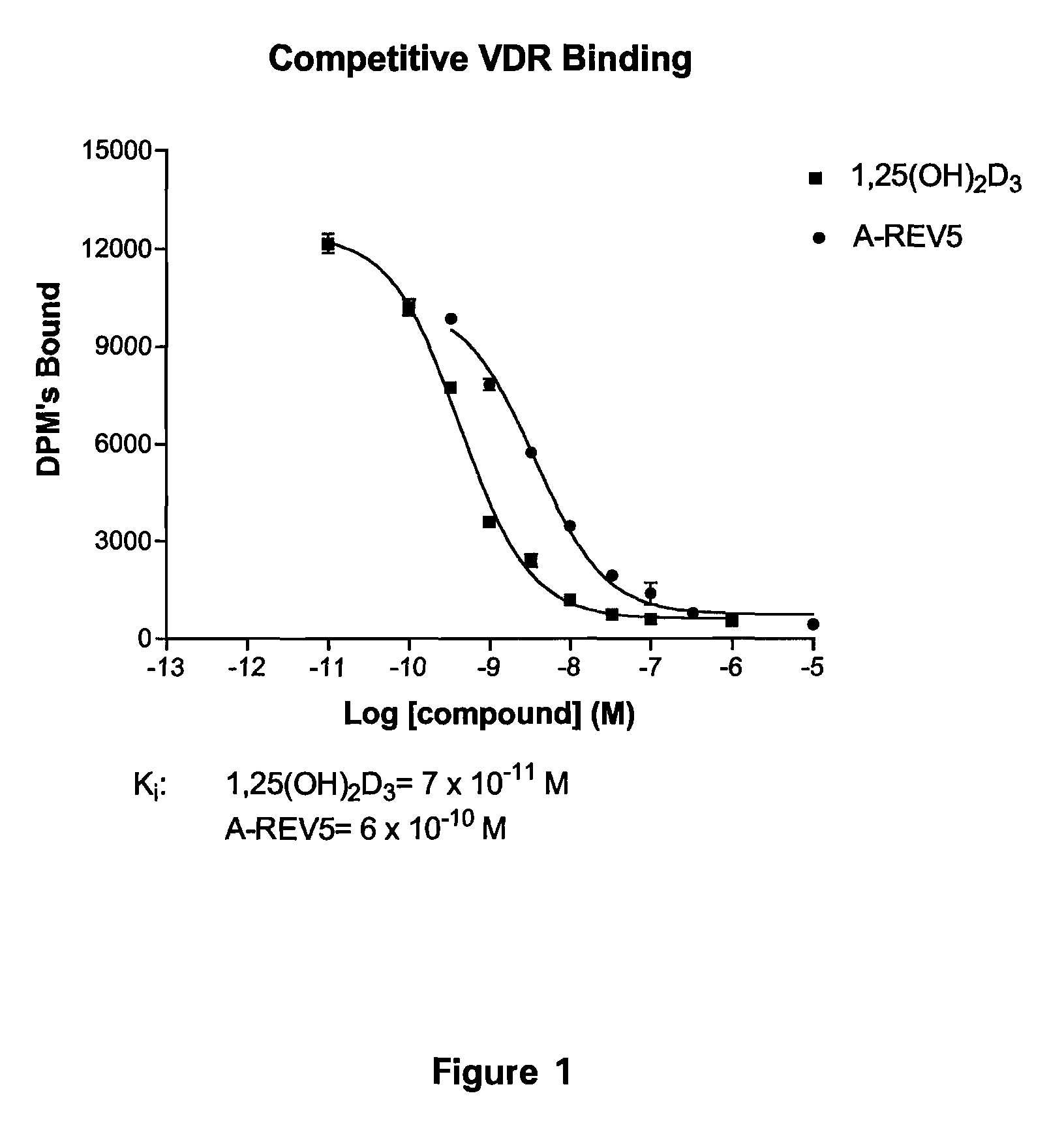
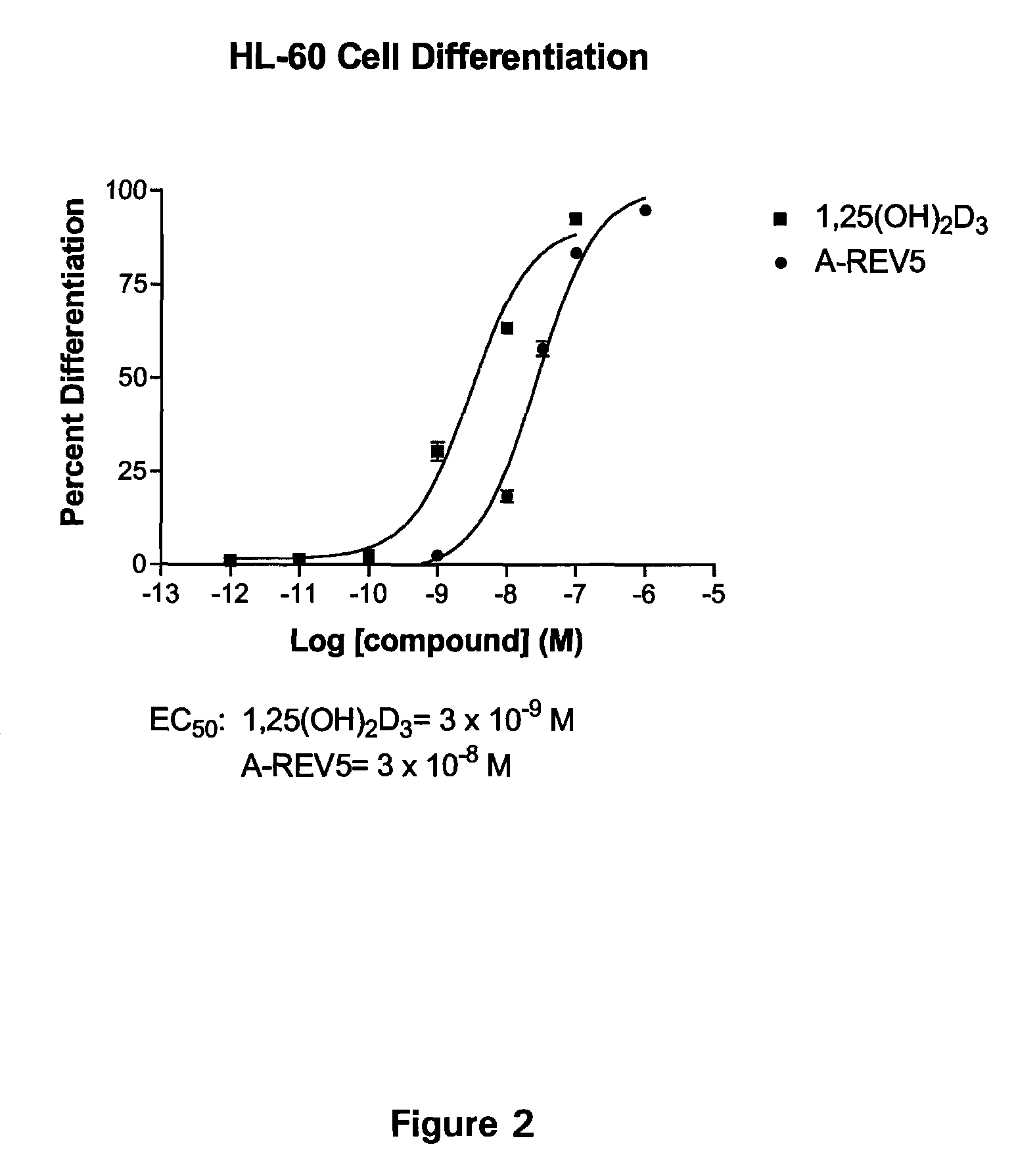
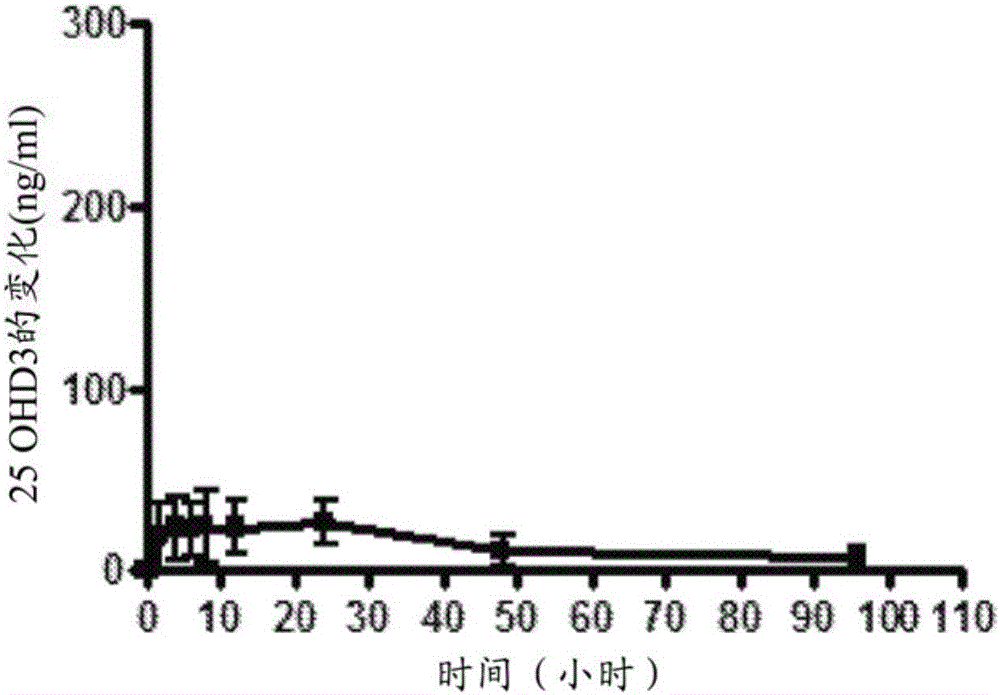
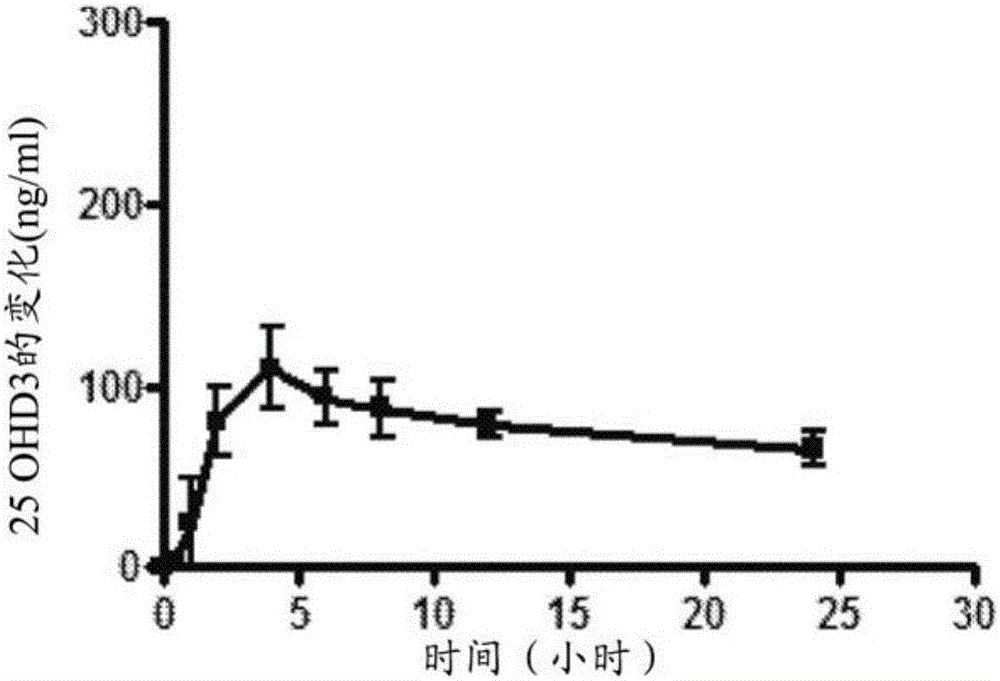
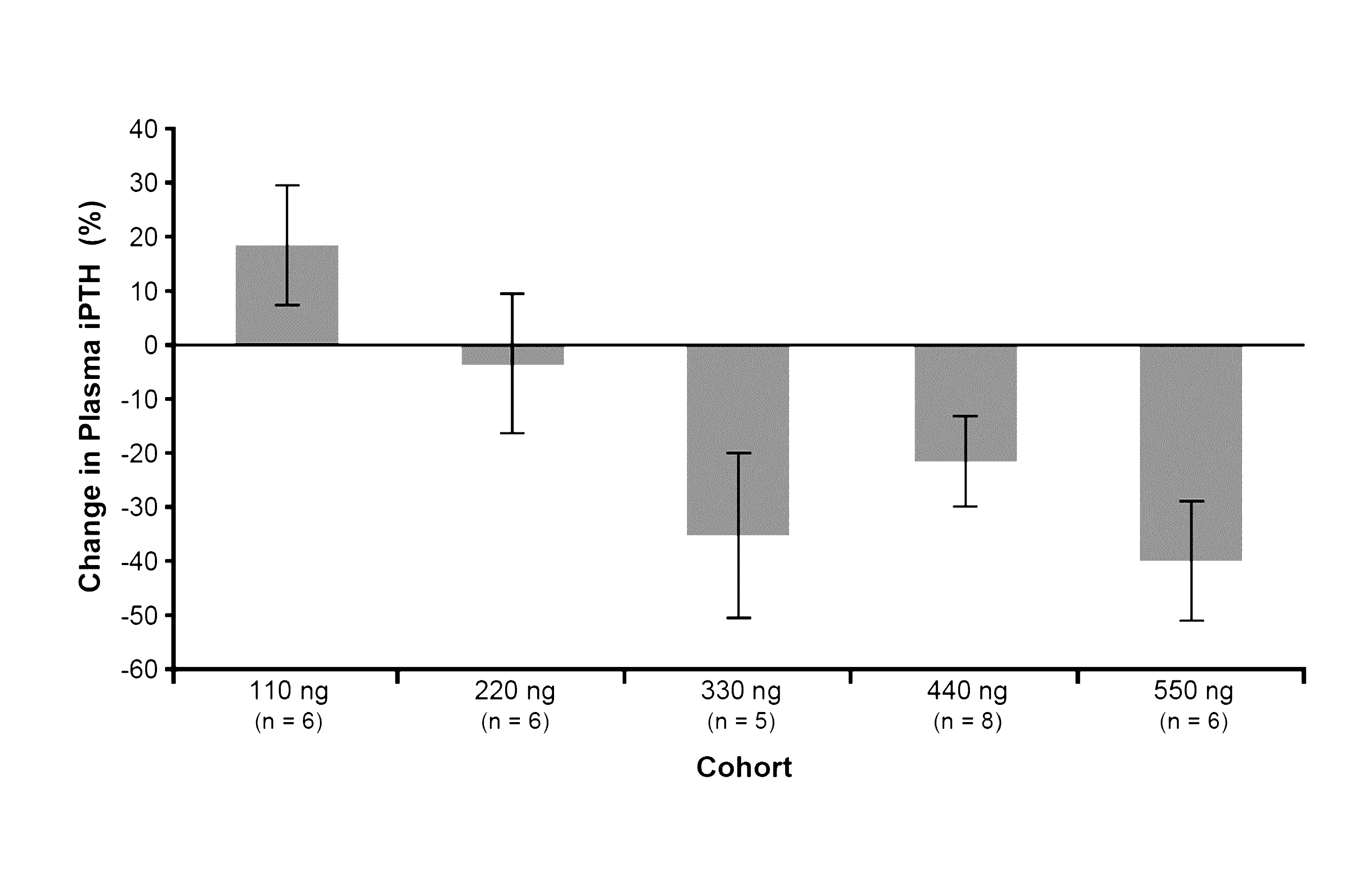
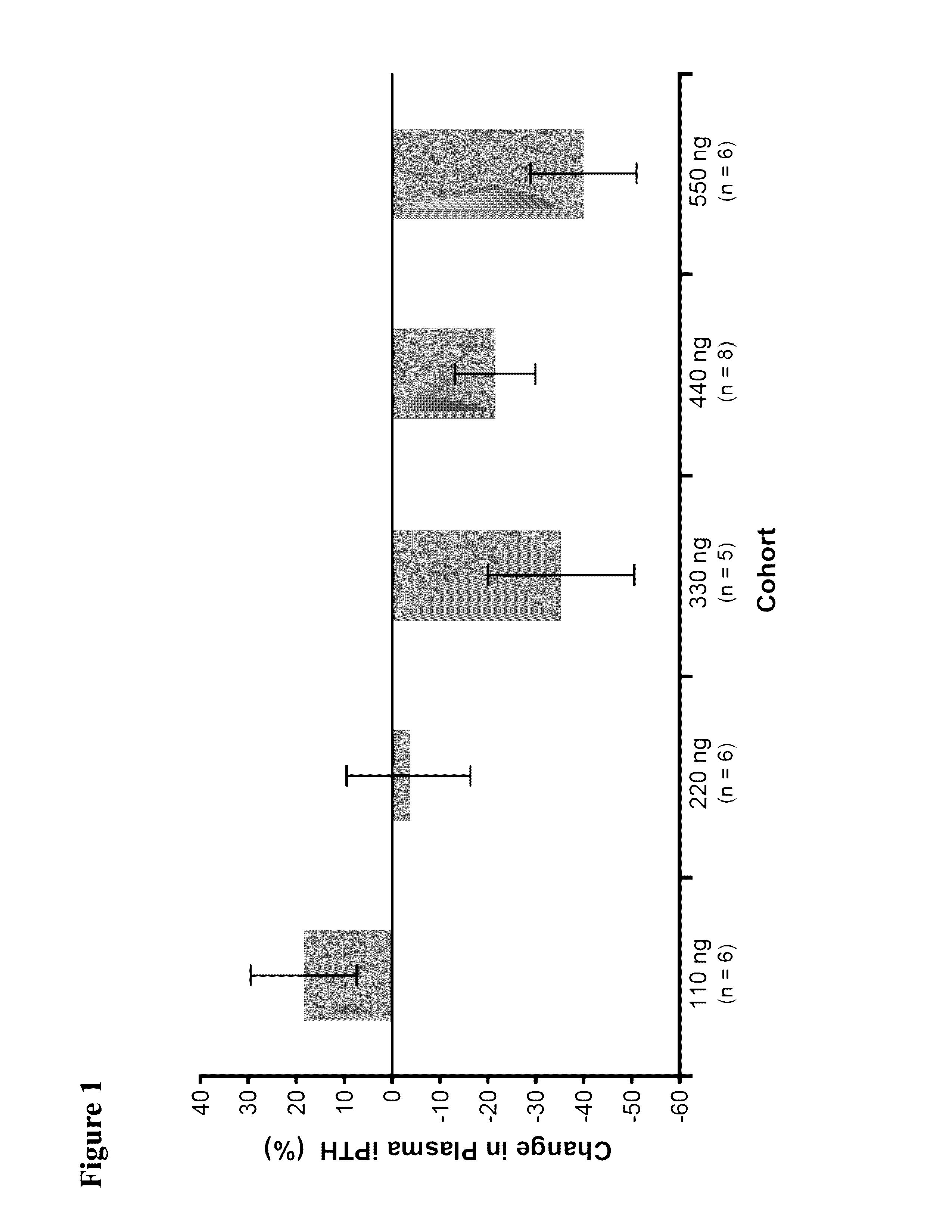
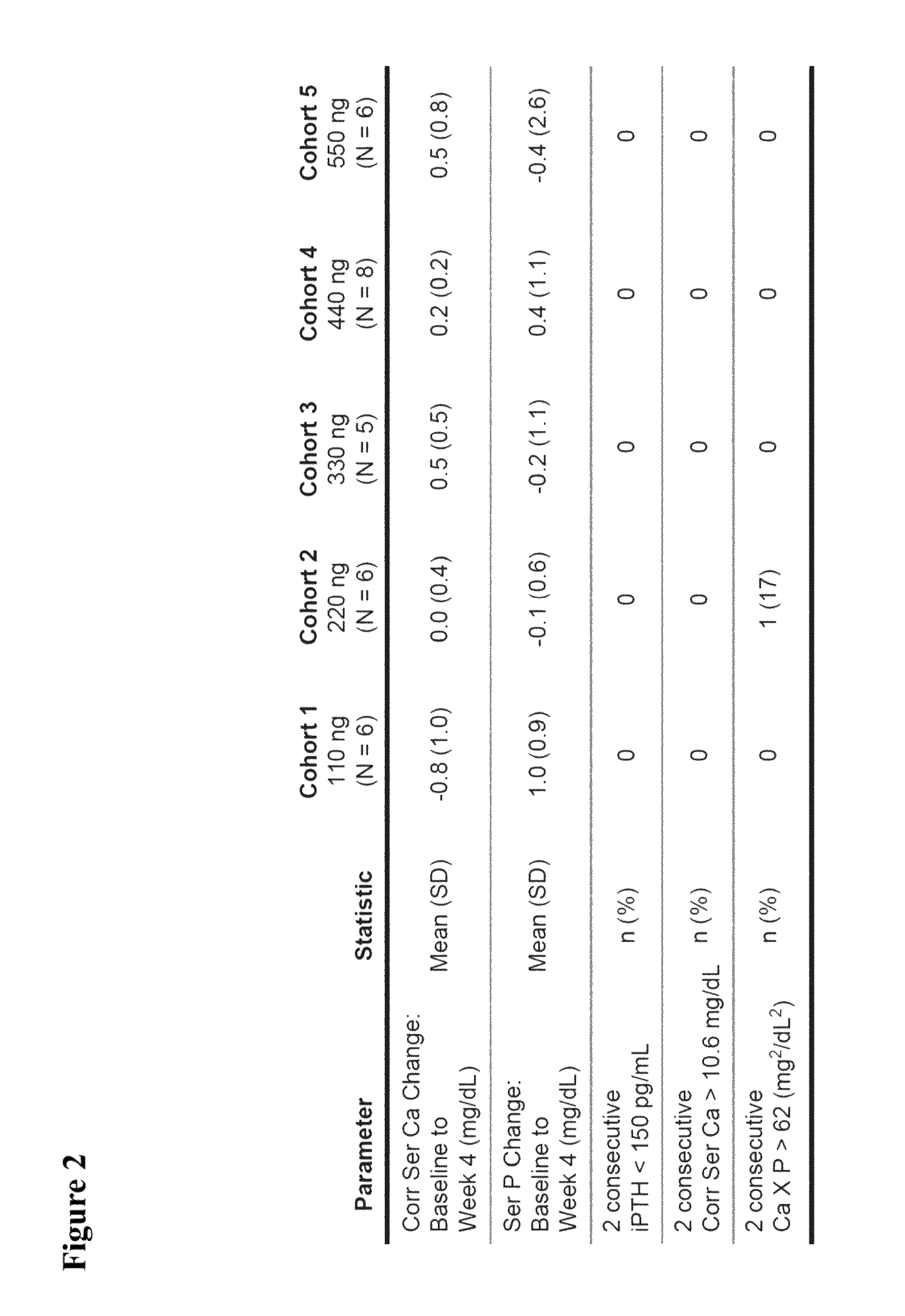
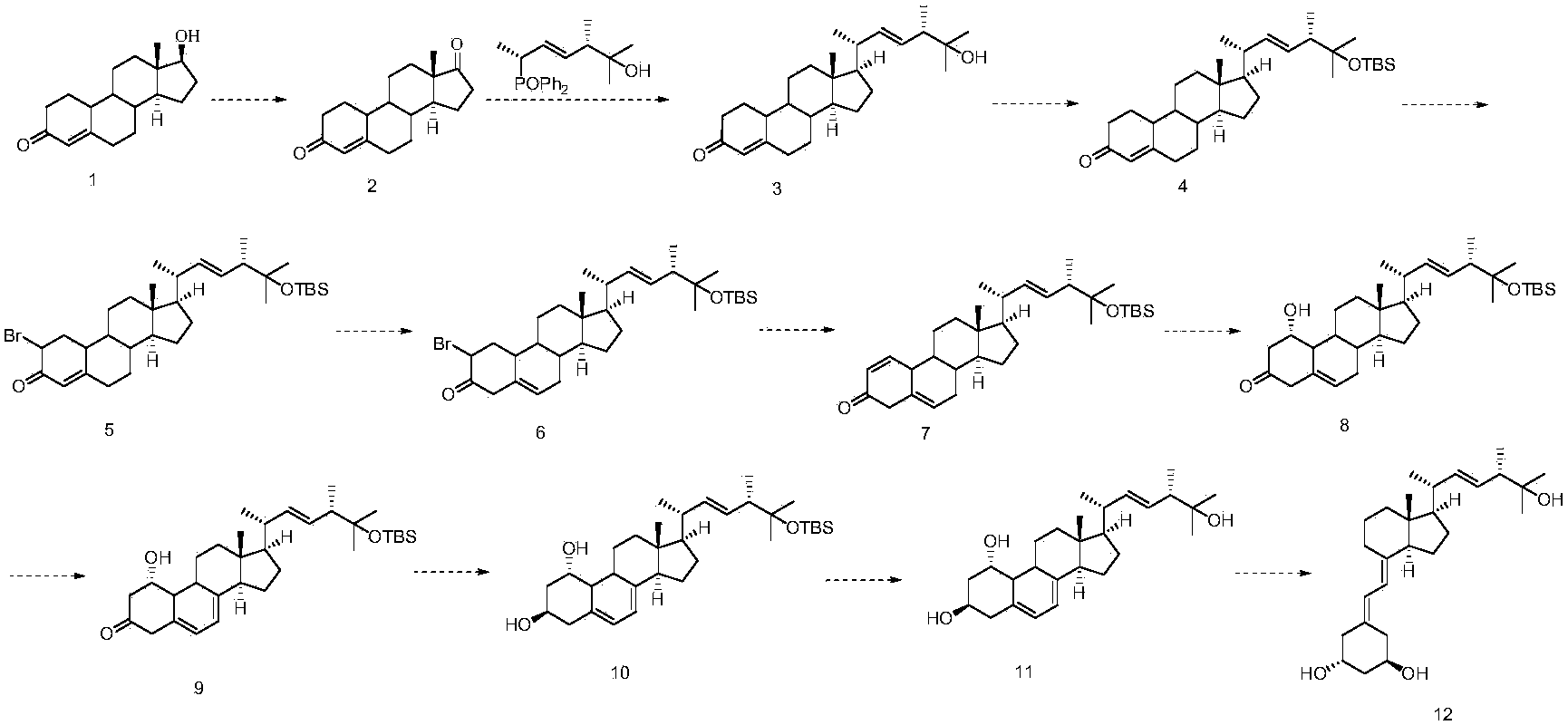
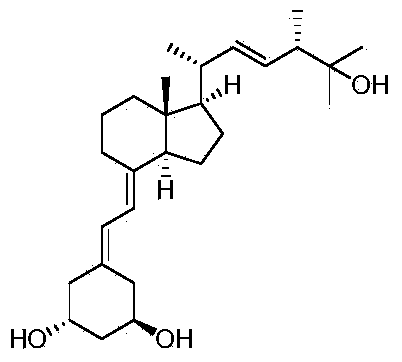
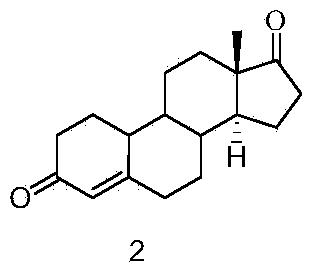
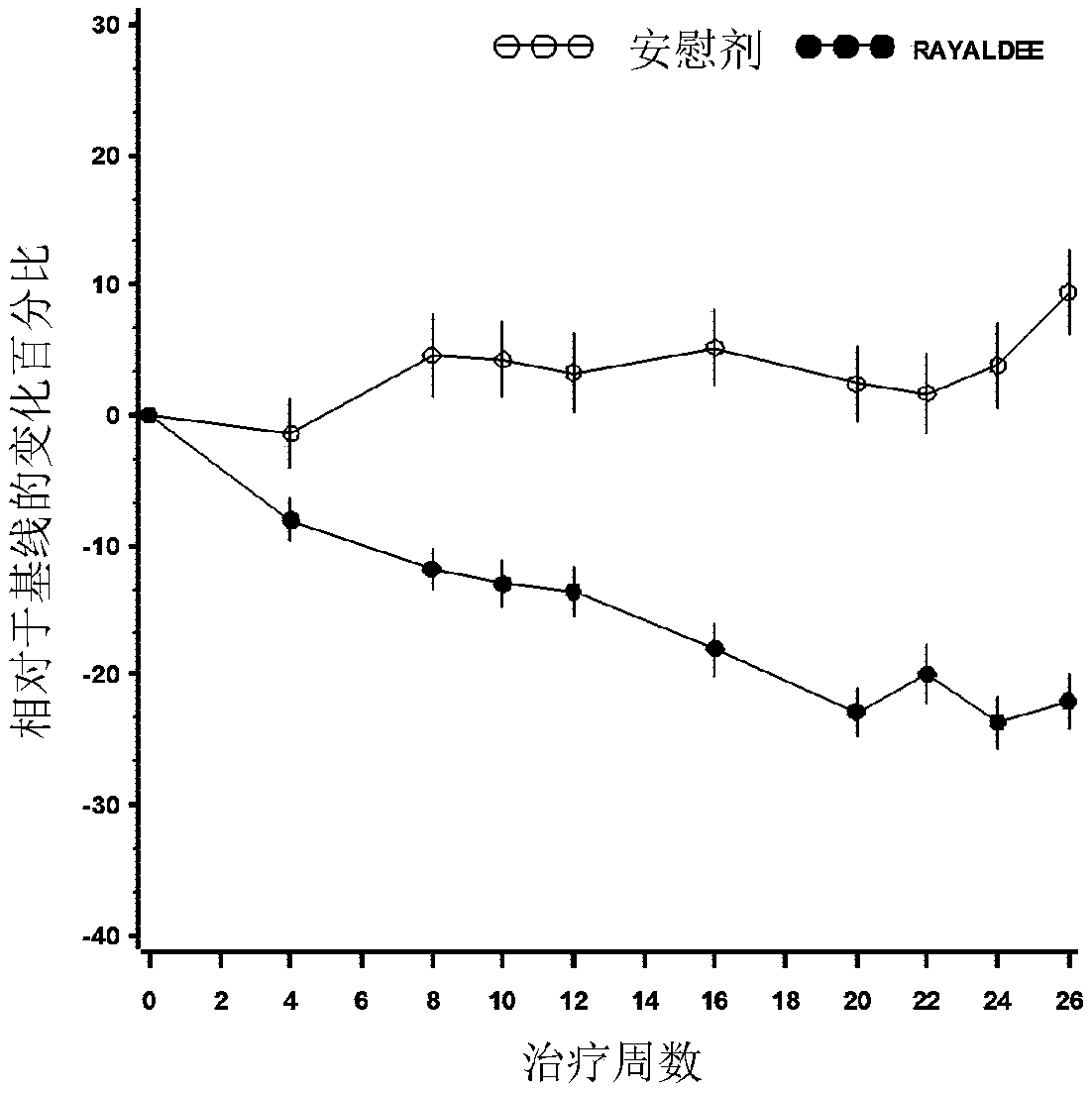
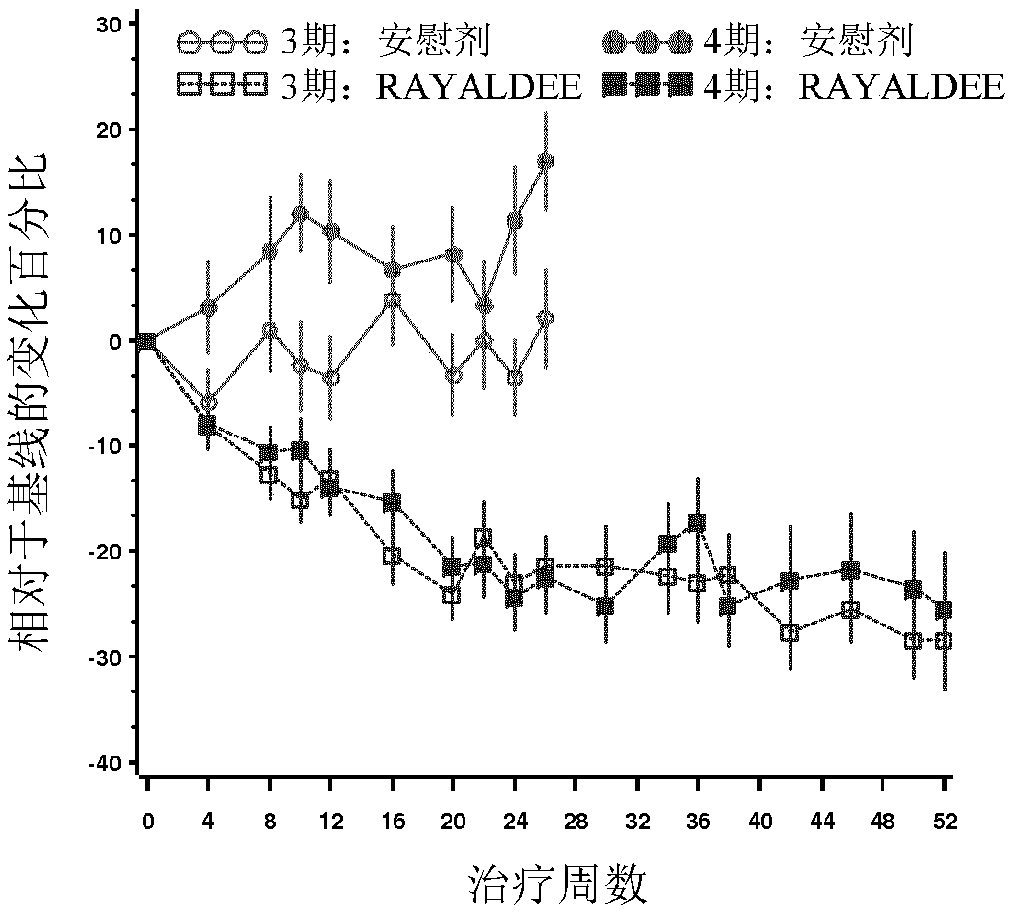
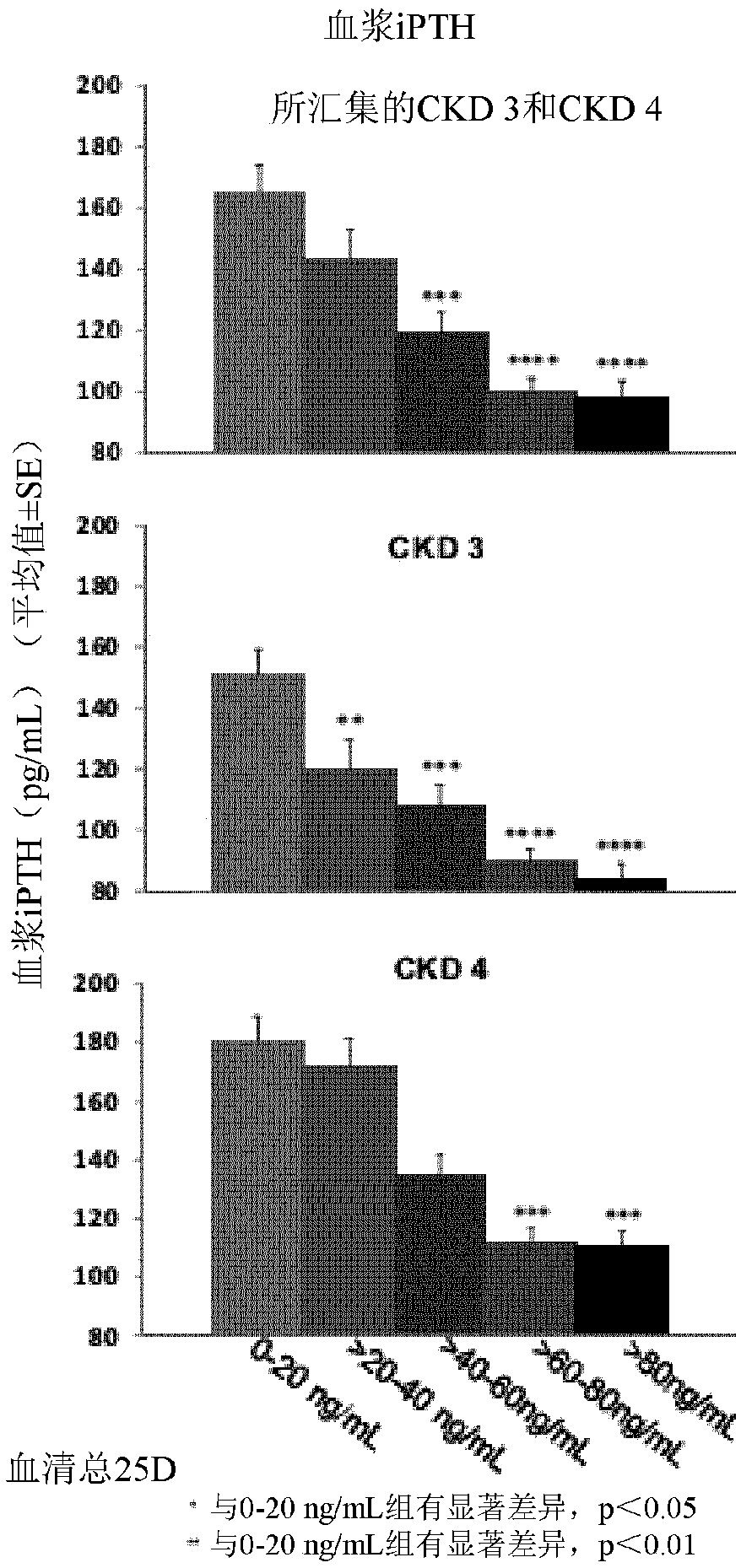
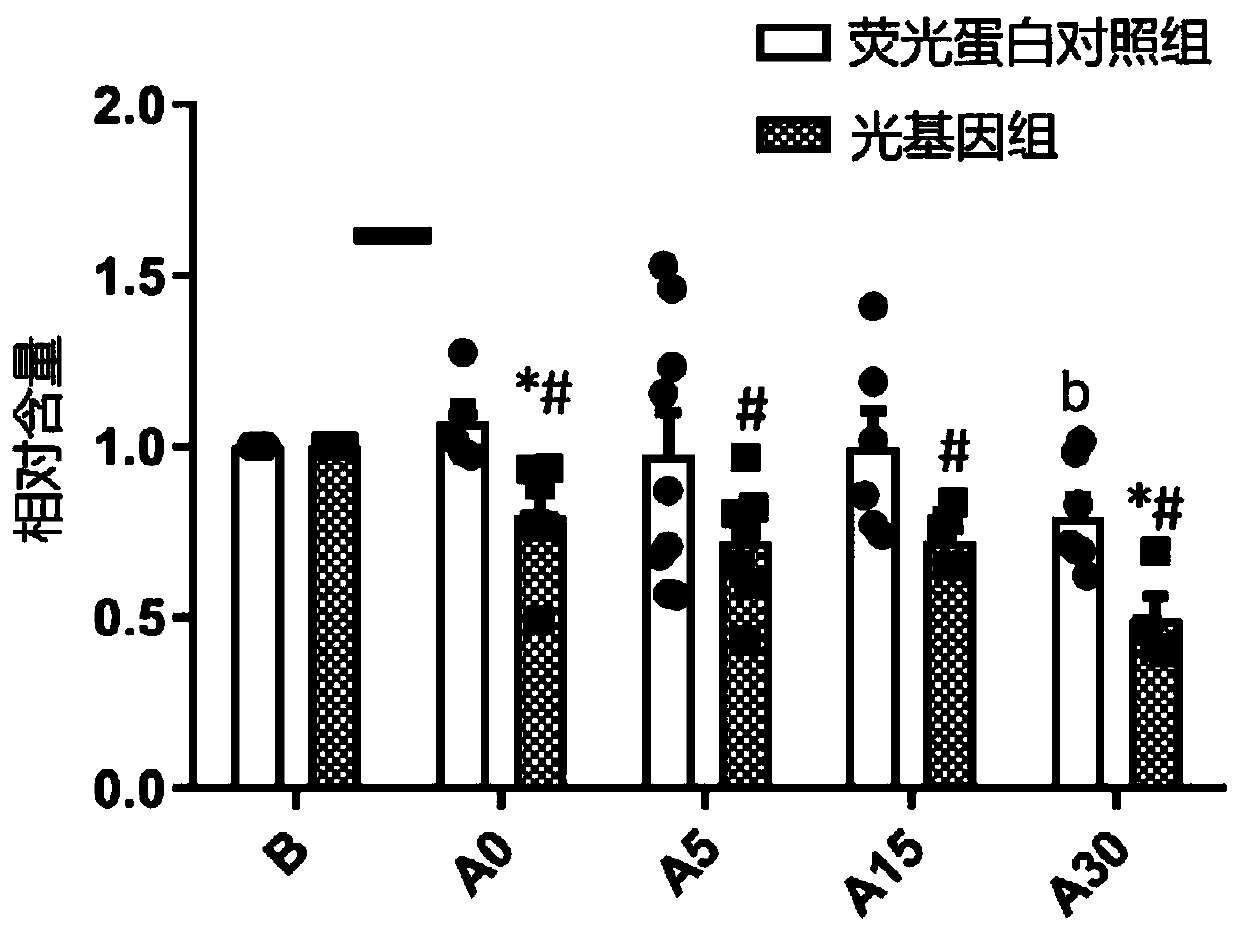
Patents
Literature
42 results about "Secondary hypoparathyroidism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
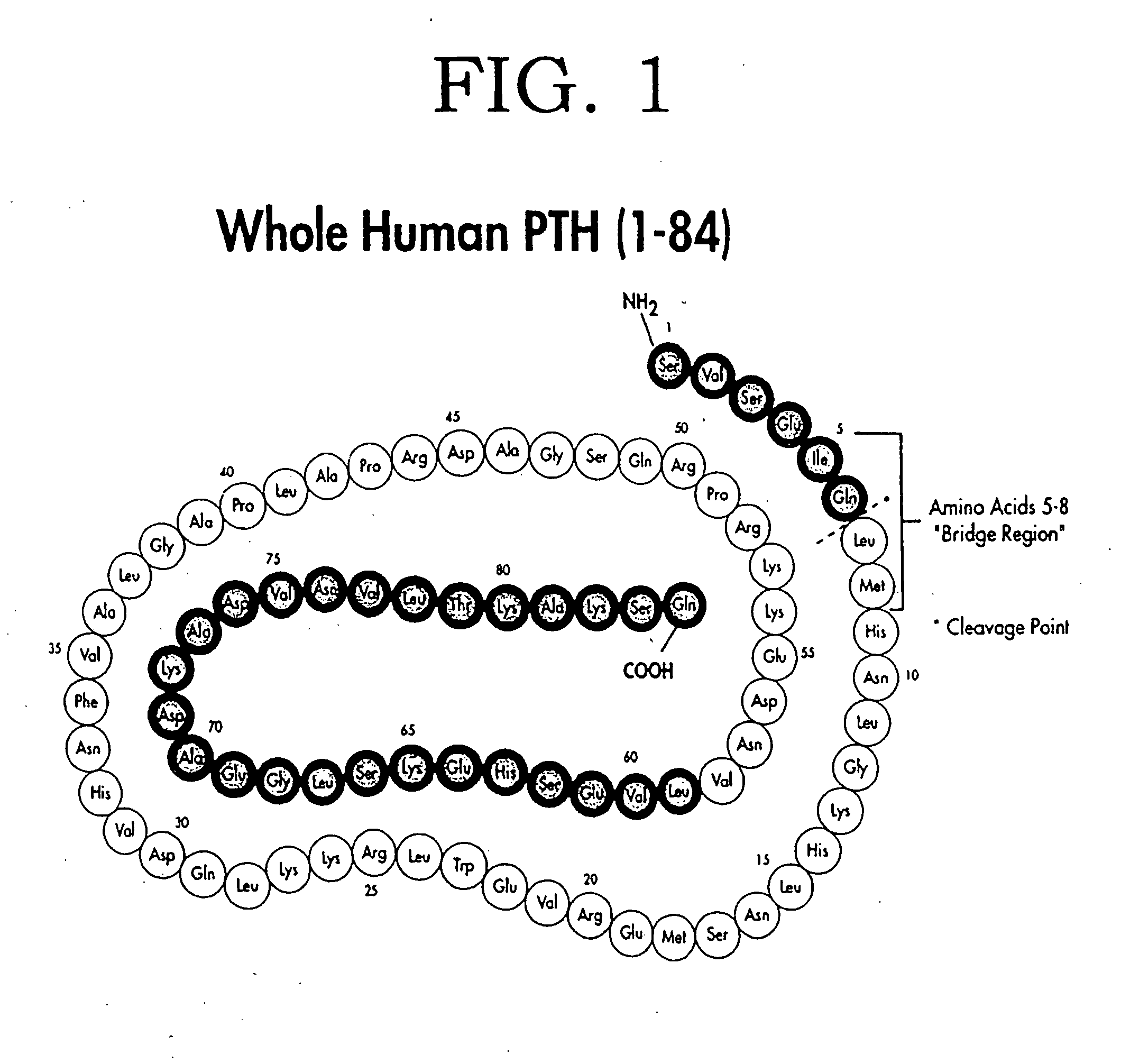
Secondary hyperparathyroidism (SHPT) refers to the excessive secretion of parathyroid hormone (PTH) by the parathyroid glands in response to hypocalcemia (low blood calcium levels) and associated hyperplasia of the glands.
Pthr1 receptor compounds
InactiveUS20110294738A1Promote absorptionNervous disorderPeptide/protein ingredientsOsteoblastSecondary hyperparathyroidism
The invention relates generally to compounds which are allosteric modulators (e.g., negative and positive allosteric modulators, allosteric agonists, and ago-allosteric modulators) of the G protein coupled receptor PTHR1, also known as parathyroid hormone / parathyroid hormone related protein receptor. The PTHR1 compounds are derived from the intracellular loops and domains of the PTHR1 receptor. The invention also relates to the use of these PTHR1 receptor compounds and pharmaceutical compositions comprising the PTHR1 receptor compounds in the treatment of diseases and conditions associated with PTHR1 receptor modulation, such as osteoporosis; humoral hypercalcemia of malignancy; osteolytic and osteoblastic metastasis to bone; primary and secondary hyperparathyroidism associated increase in bone absorption; vascular calcification; psychiatric disorders and cognitive disorders associated with hyperparathyroidism; dermatological disorders; and excess hair growth.
Owner:REN YONG +2
Methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism
Owner:SCANTIBODIES LAB
Methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism
InactiveUS7056655B2Lower Level RequirementsMinimises levelOrganic active ingredientsBiocideNephrosisEprotirome
The present invention relates to novel methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism. One determines and monitors the level of parathyroid hormone agonist and parathyroid hormone antagonist in the renal patient. The parathyroid hormone suppressing therapeutic is administered to the patient so as to minimize the level of parathyroid hormone antagonist.
Owner:SCANTIBODIES LAB
Method of Safely and Effectively Treating and Preventing Secondary Hyperparathyroidism in Chronic Kidney Disease
InactiveUS20100144684A1Safe and effective usePrevent relapseBiocideOrganic active ingredientsBlood concentrationHormone replacement
A method of treating and preventing secondary hyperparathyroidism in CKD by increasing or maintaining blood concentrations of both 25-hydroxyvitamin D and 1,25-dihydroxyvitamin Din a patient by administering 25-hydroxyvitamin D3 with or without 25-hydroxyvitamin D2 and, as necessary, 1,25-dihydroxyvitamin D2 as a Vitamin D hormone replacement therapy.
Owner:OPKO RENAL LLC
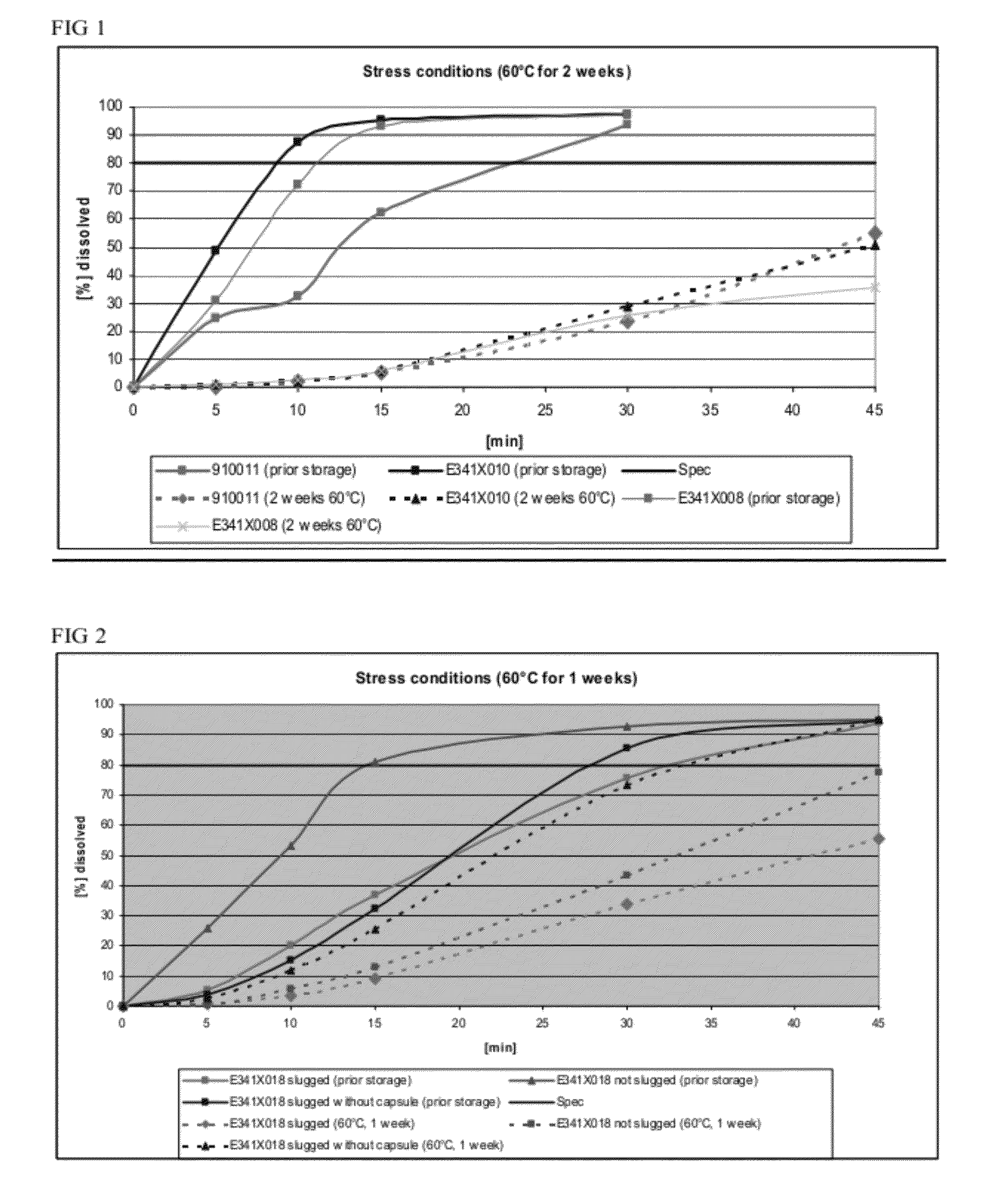
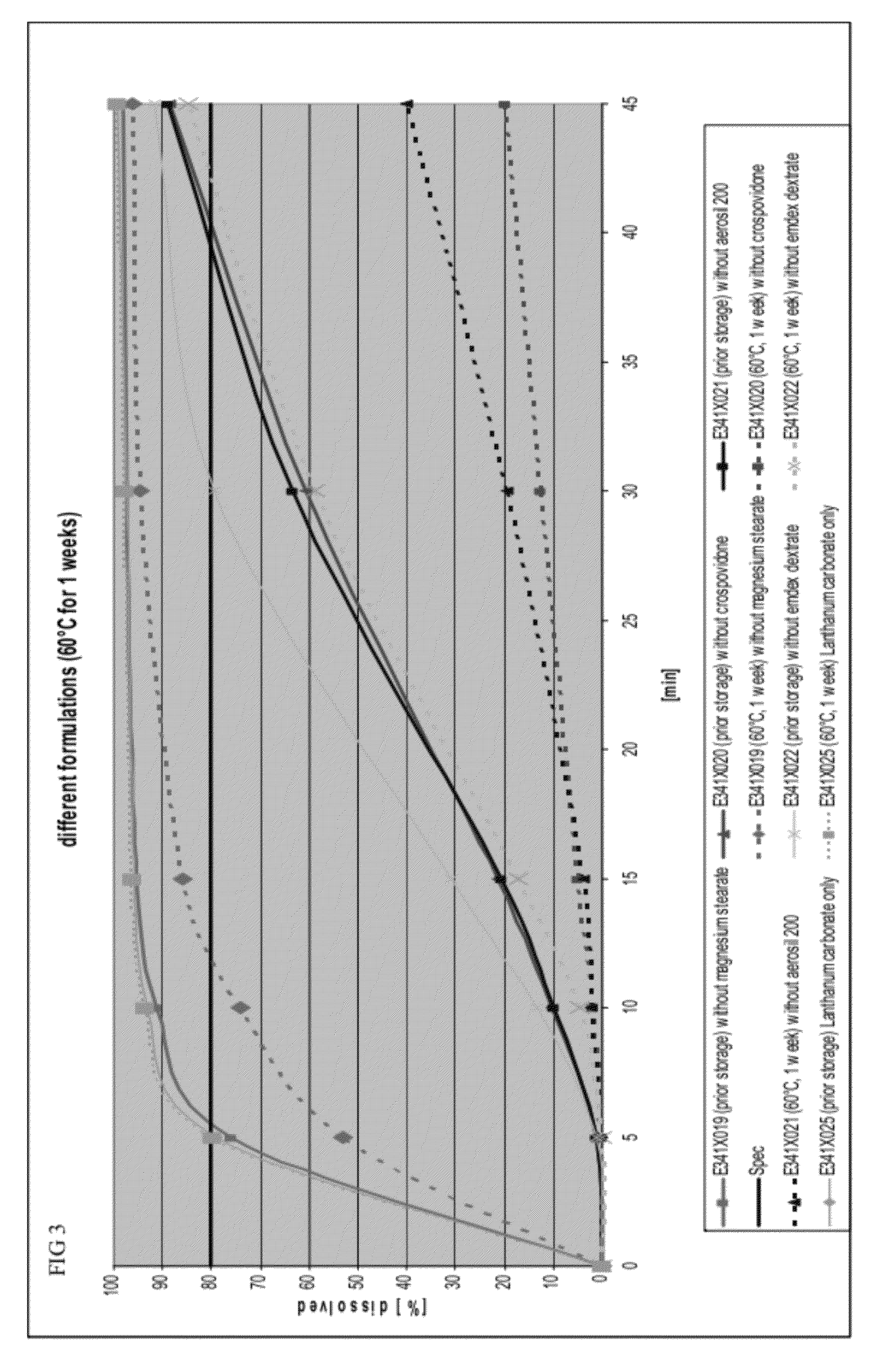
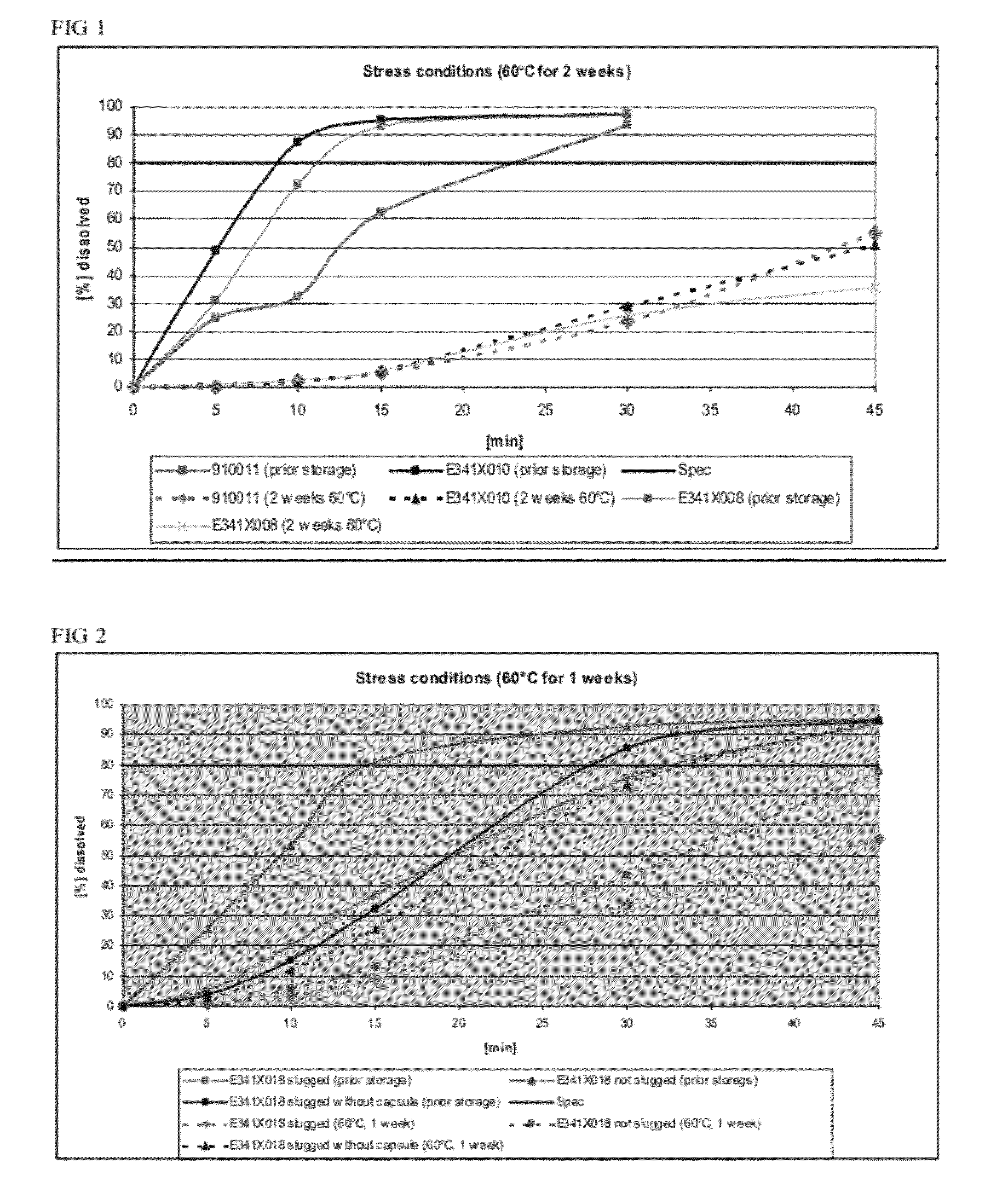
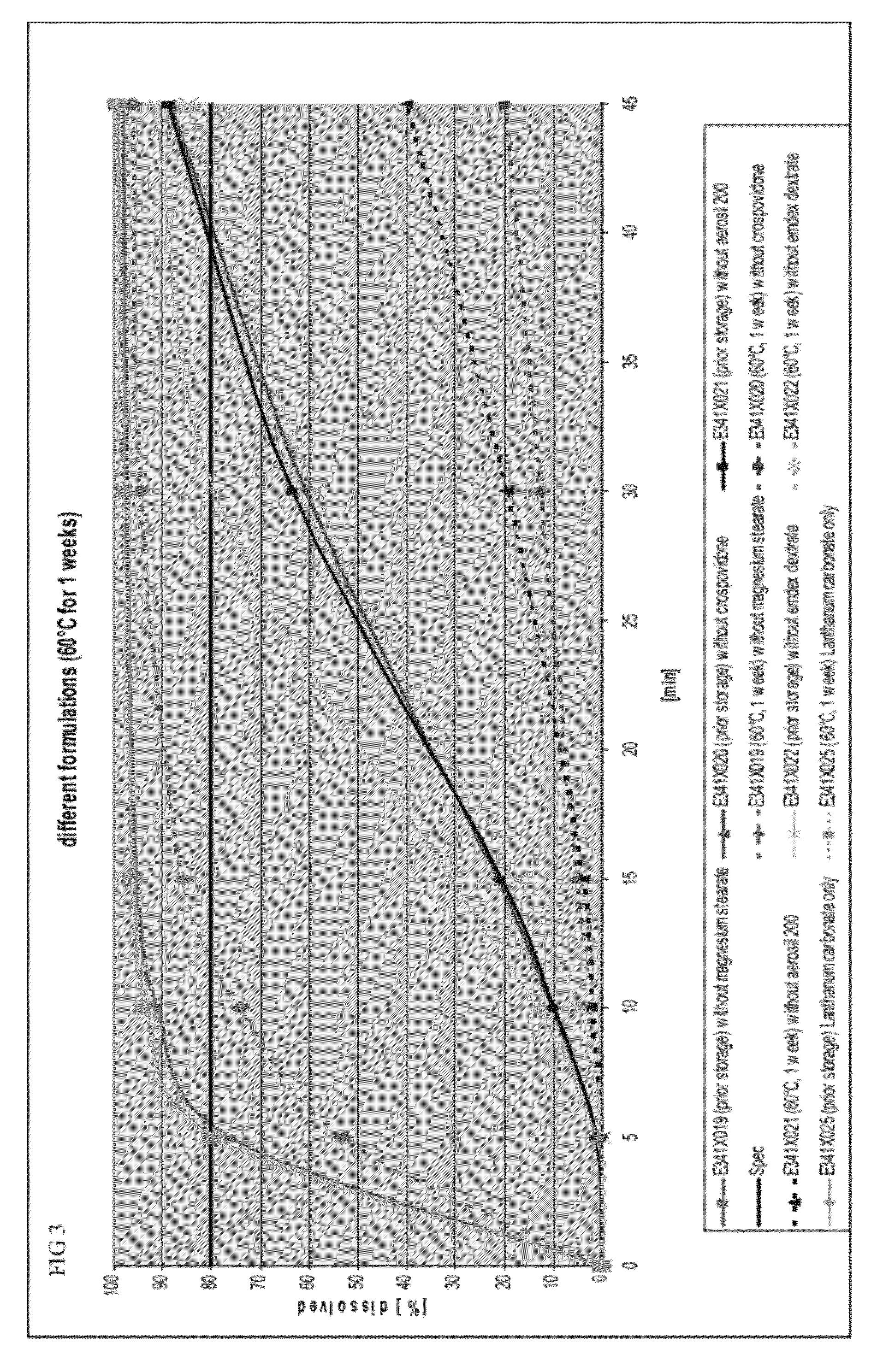
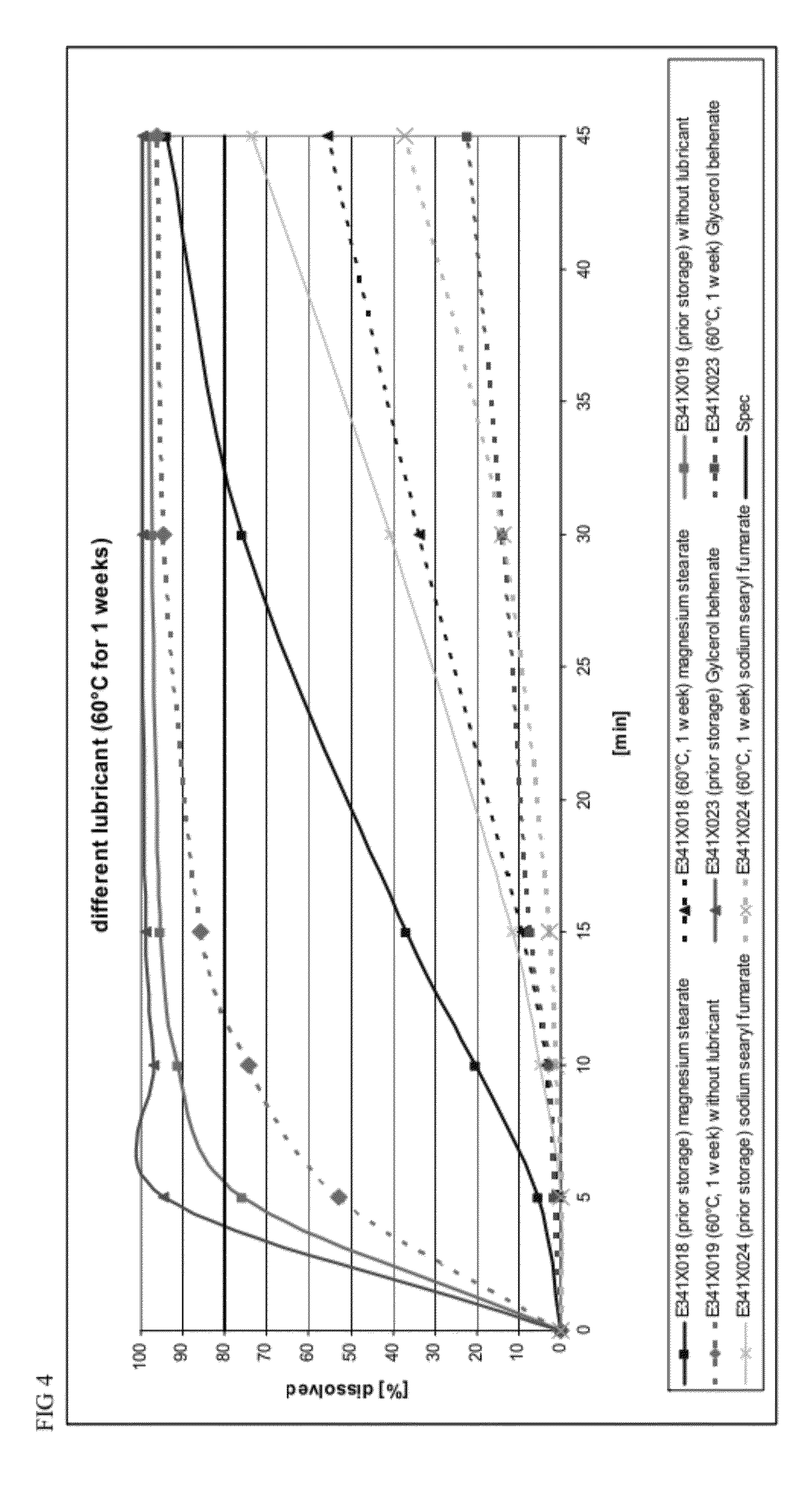
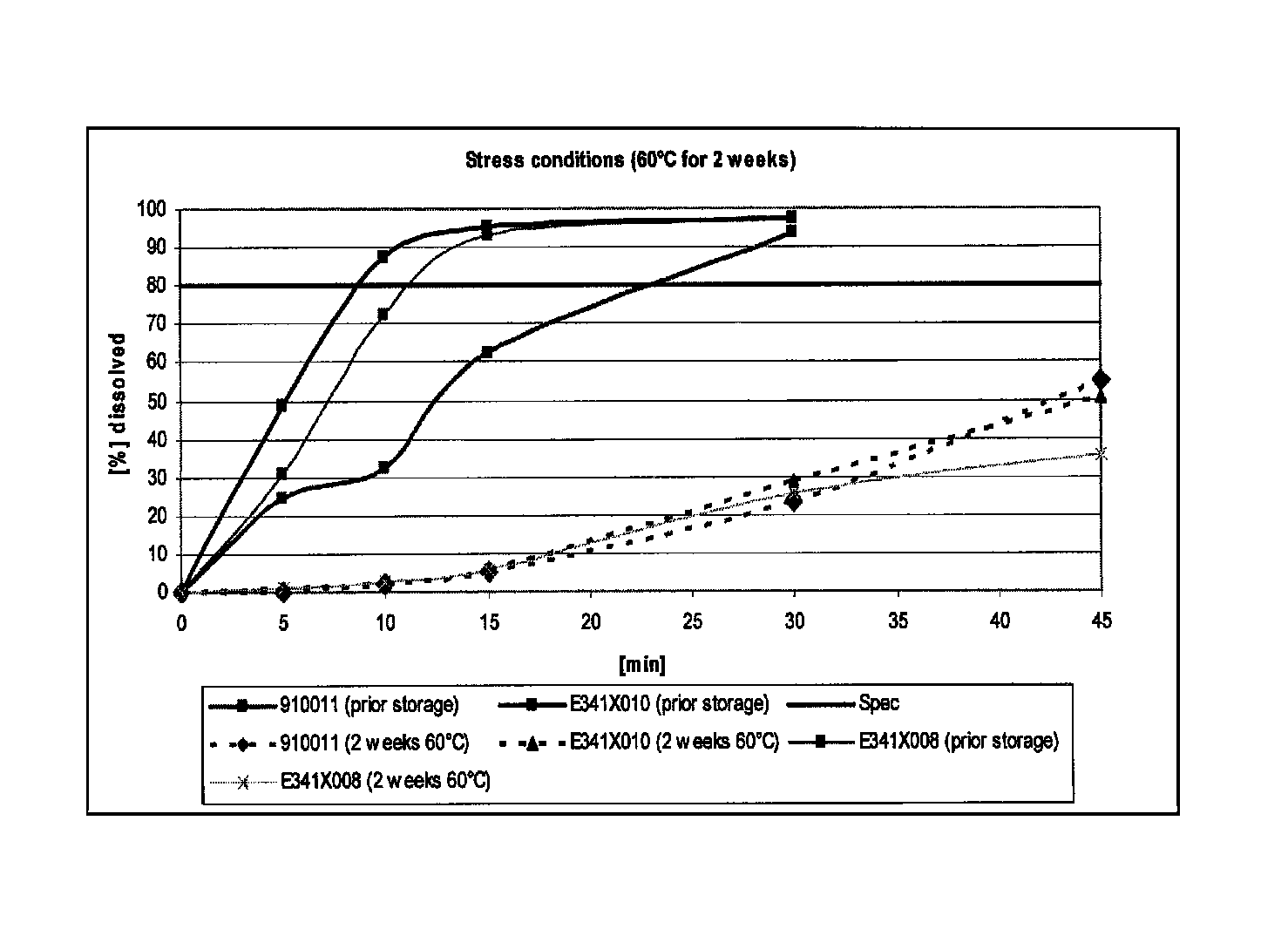
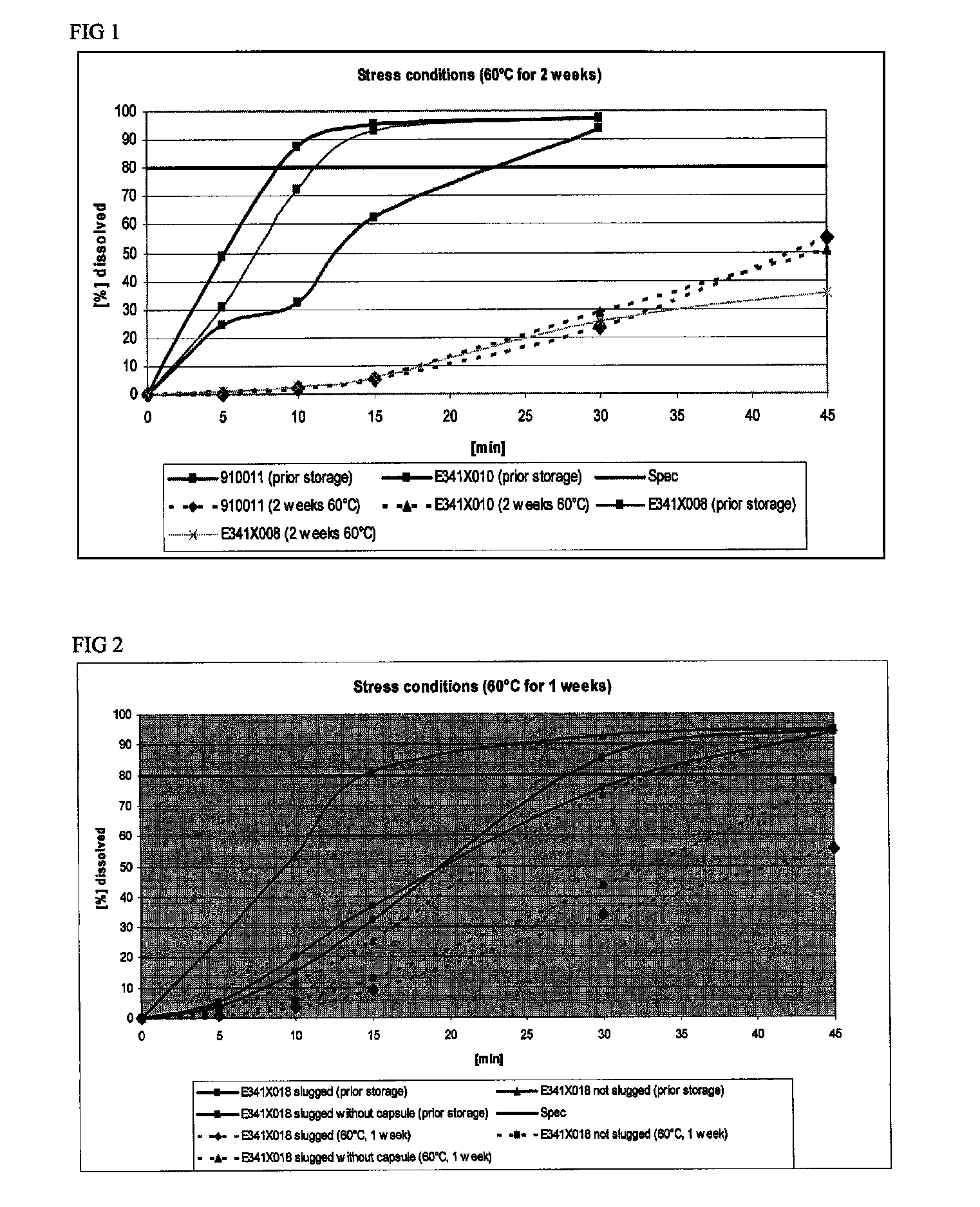
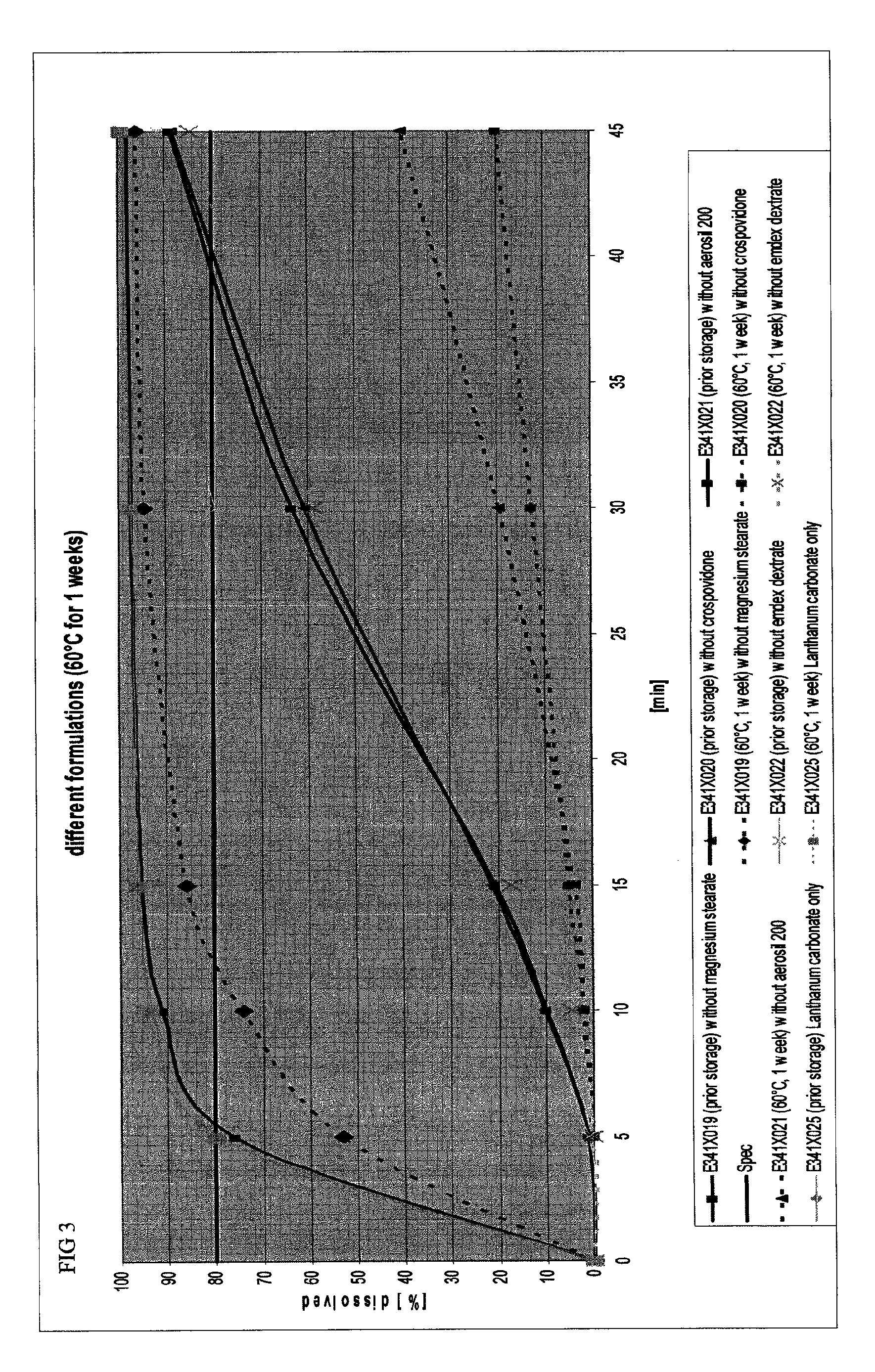
Capsule formulations containing lanthanum compounds
The present invention includes an oral pharmaceutical capsule comprising a shell, lanthanum carbonate or lanthanum carbonate hydrate, and a lubricant such as talc, wherein the shell encapsulates the lanthanum carbonate or lanthanum hydrate and the lubricant. Capsule shells comprise, for example, gelatin. The capsules of the present invention dissolve at a similar rate before and after storage. The oral pharmaceutical capsules of the present invention can be administered to treat a patient at risk for or suffering from hyperphosphatemia, at risk for or suffering from chronic kidney disease (CKD), at risk for or suffering from soft tissue calcification associated with CKD, or at risk for or suffering from secondary hyperparathyroidism.
Owner:SHIRE PLC
Method of safely and effectively treating and preventing secondary hyperparathyroidism in chronic kidney disease
InactiveUS20140274977A1Organic active ingredientsBiocideBlood concentrationSecondary hyperparathyroidism
Owner:OPKO RENAL LLC
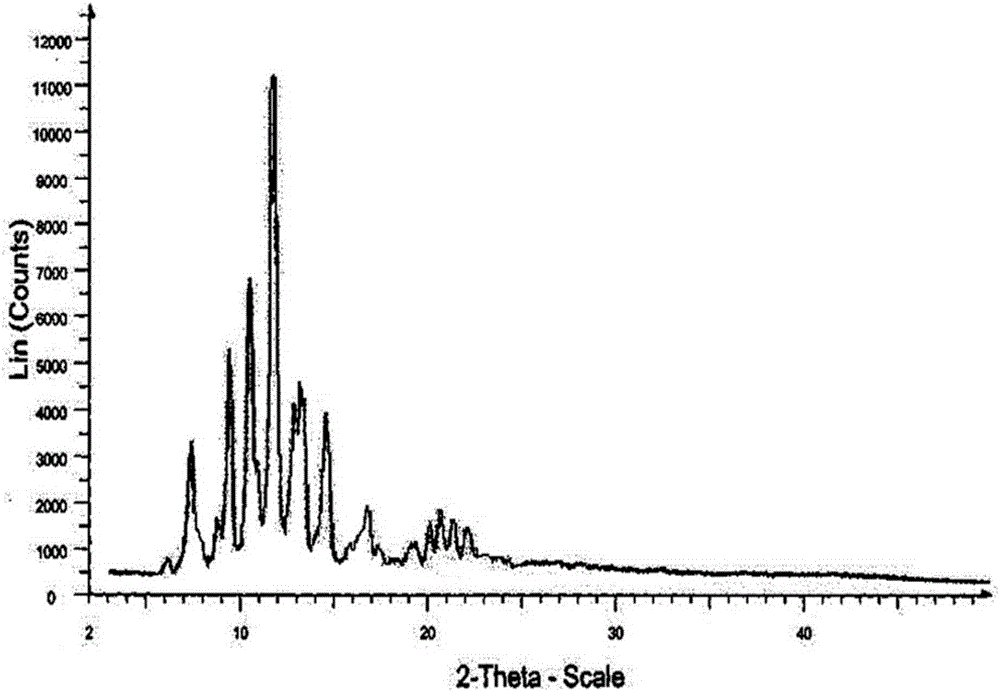
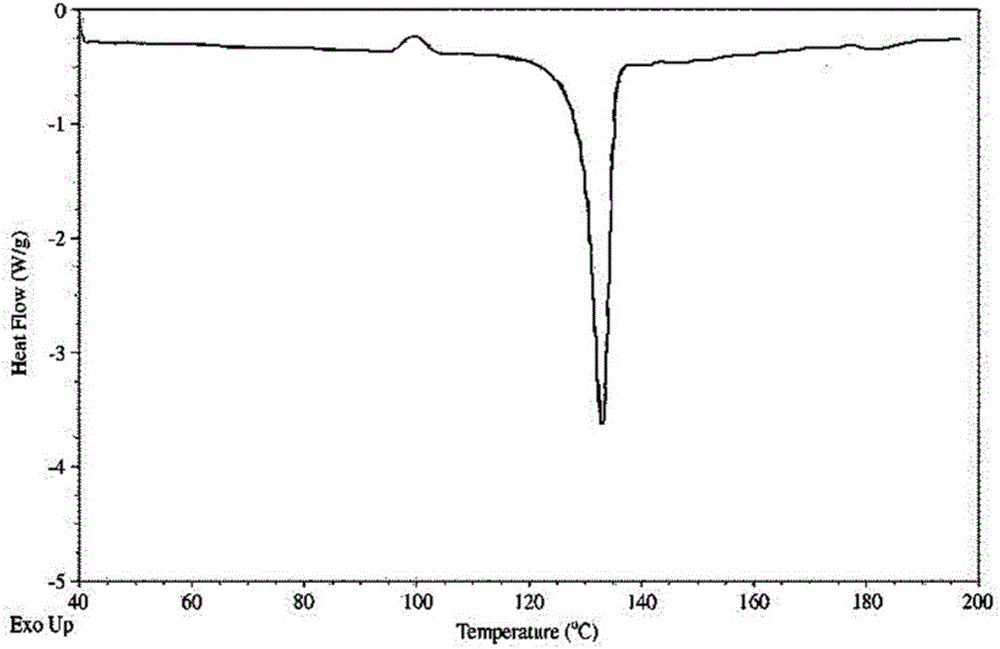
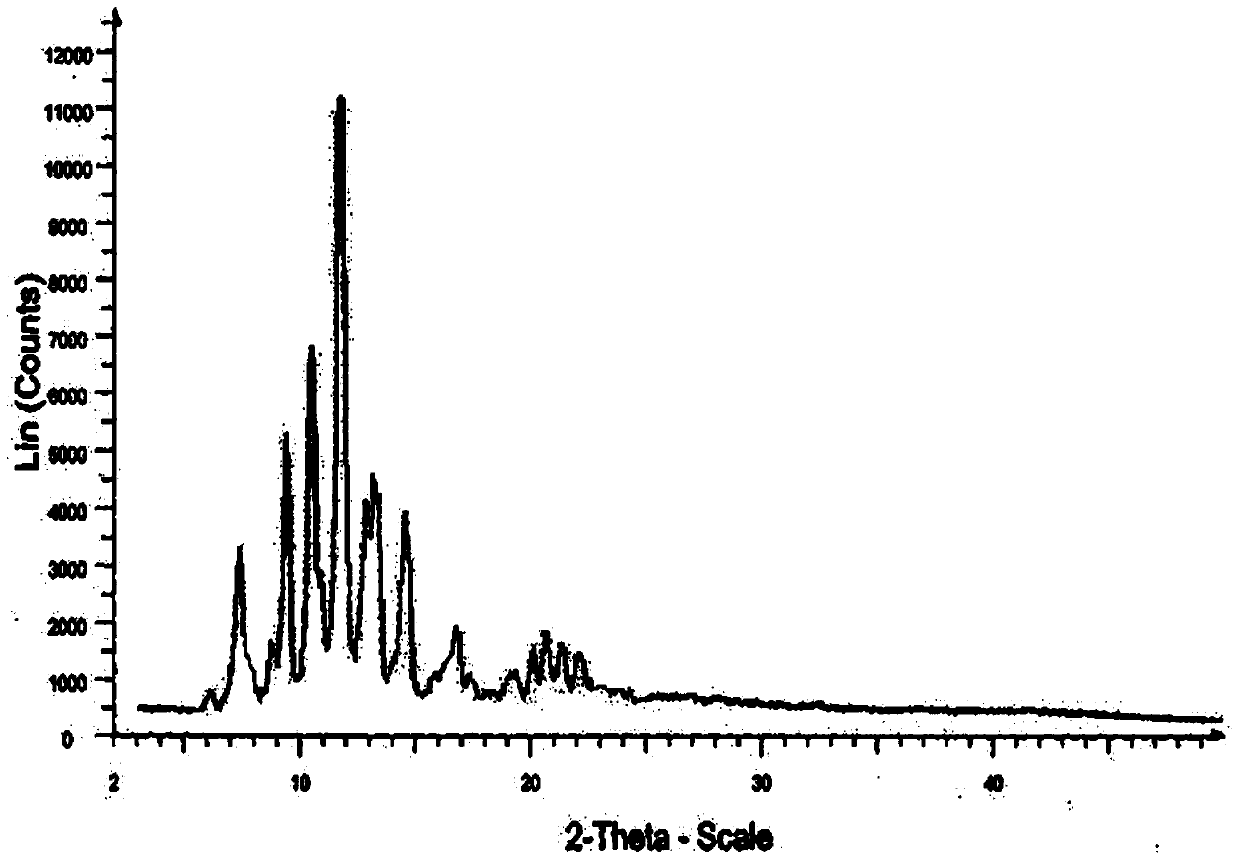
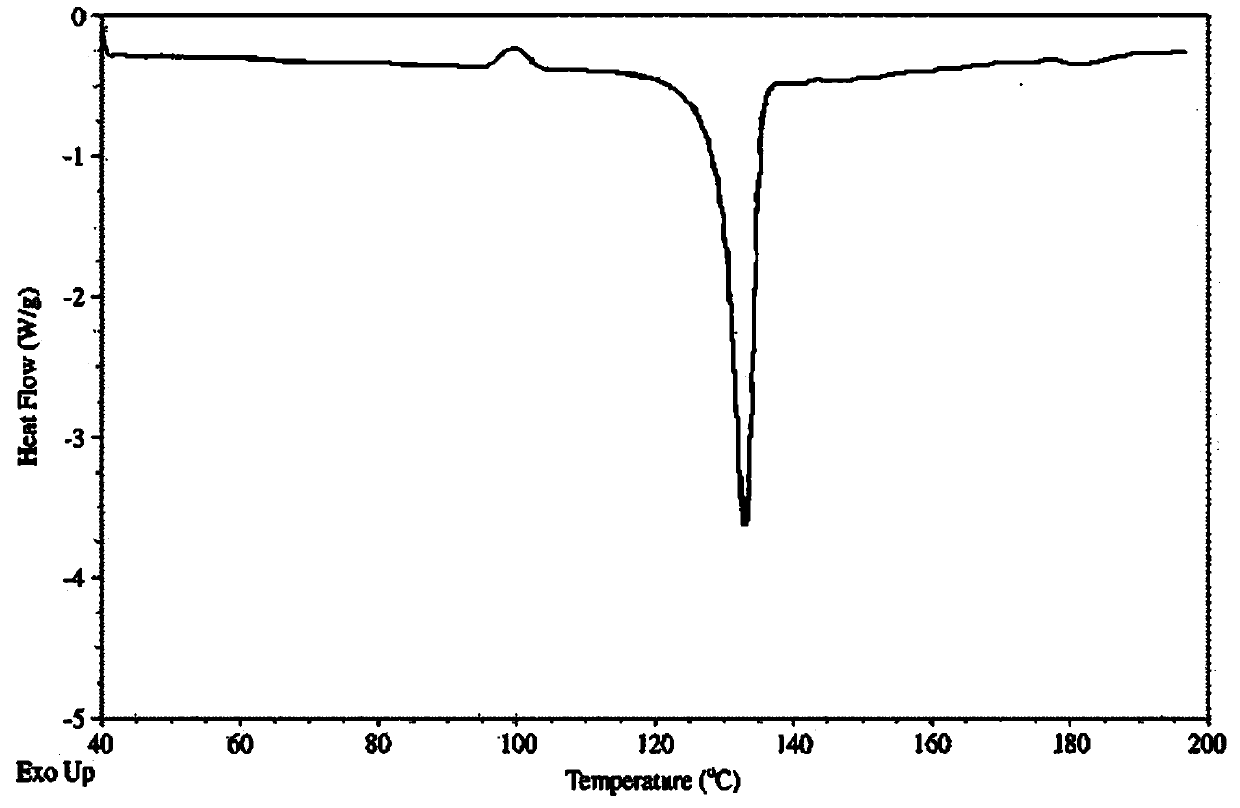
Novel crystalline form of doxercalciferol and preparation method for novel crystalline form
ActiveCN105237452AGood physical and chemical propertiesImprove stabilityOrganic active ingredientsOrganic chemistryMedicineDoxercalciferol
The invention belongs to the field of medicinal chemistry, and particularly relates to a novel crystalline form A of doxercalciferol and a preparation method for the novel crystalline form A. The invention further comprises a pharmaceutical composition of the novel crystalline form A of doxercalciferol and an application of the composition for treating osteoporosis or secondary hyperparathyroidism. By virtue of Cu-Kalpha radiation, X-ray powder diffraction has diffraction peaks at positions where 2theta angles are 7.82, 8.51, 12.06, 13.01, 15.60, 17.05, 17.82, 18.71, 19.35, 20.06, 20.37, 20.62, 21.98, 22.68, 22.90, 23.81 and 24.79. The invention further discloses a technically improved scheme for preparing high purity doxercalciferol at the same time. The scheme is simple in step, easy to operate, short in consumed time and low in cost, and industrial large-scaled production is quite facilitated.
Owner:NANJING HERON PHARM CO LTD
Calcimimetics and methods for their use
InactiveUS20140315809A1Easy to solveDecrease PTHPeptide/protein ingredientsMetabolism disorderHemodialysisHaemodialysis machine
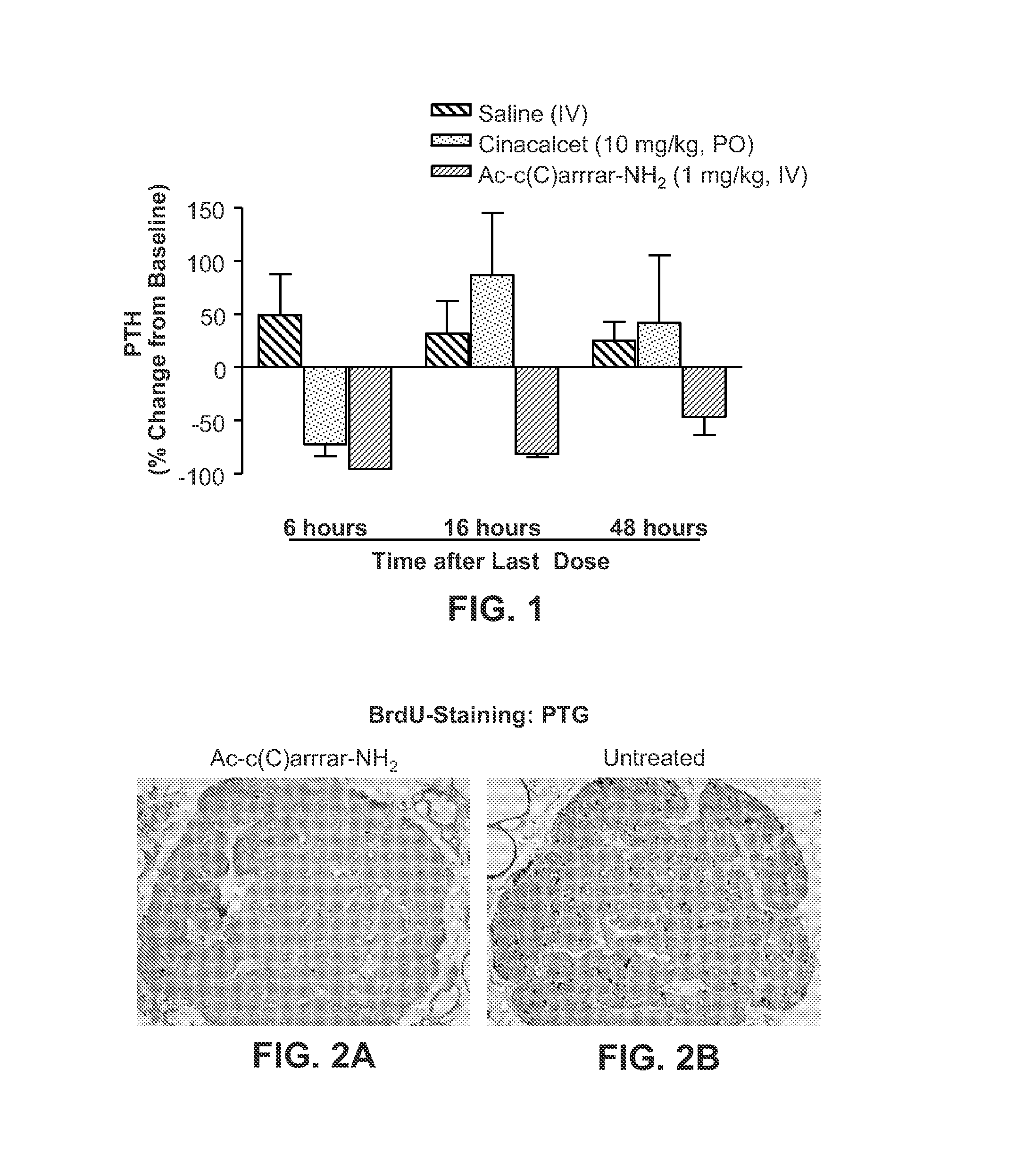
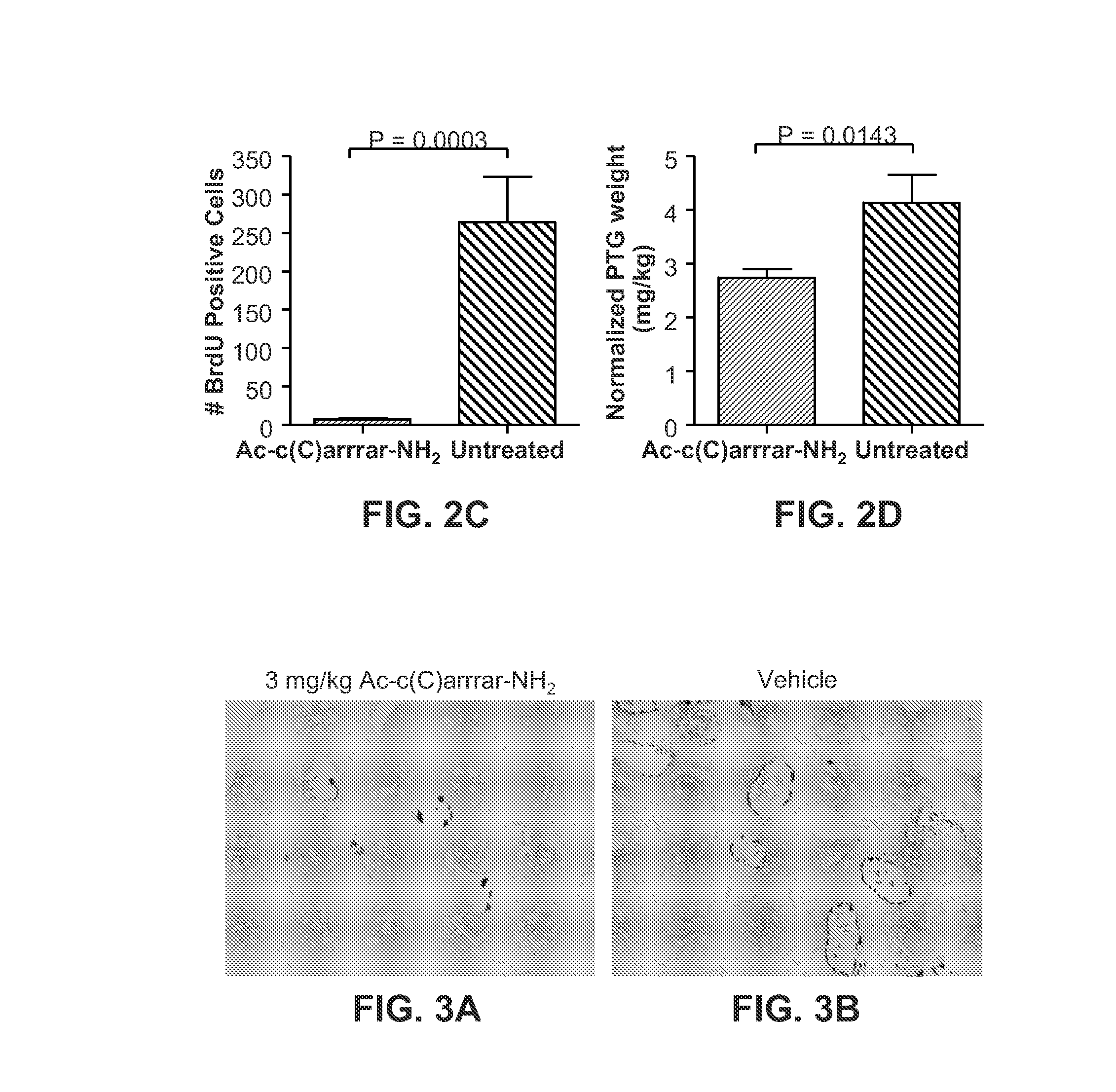
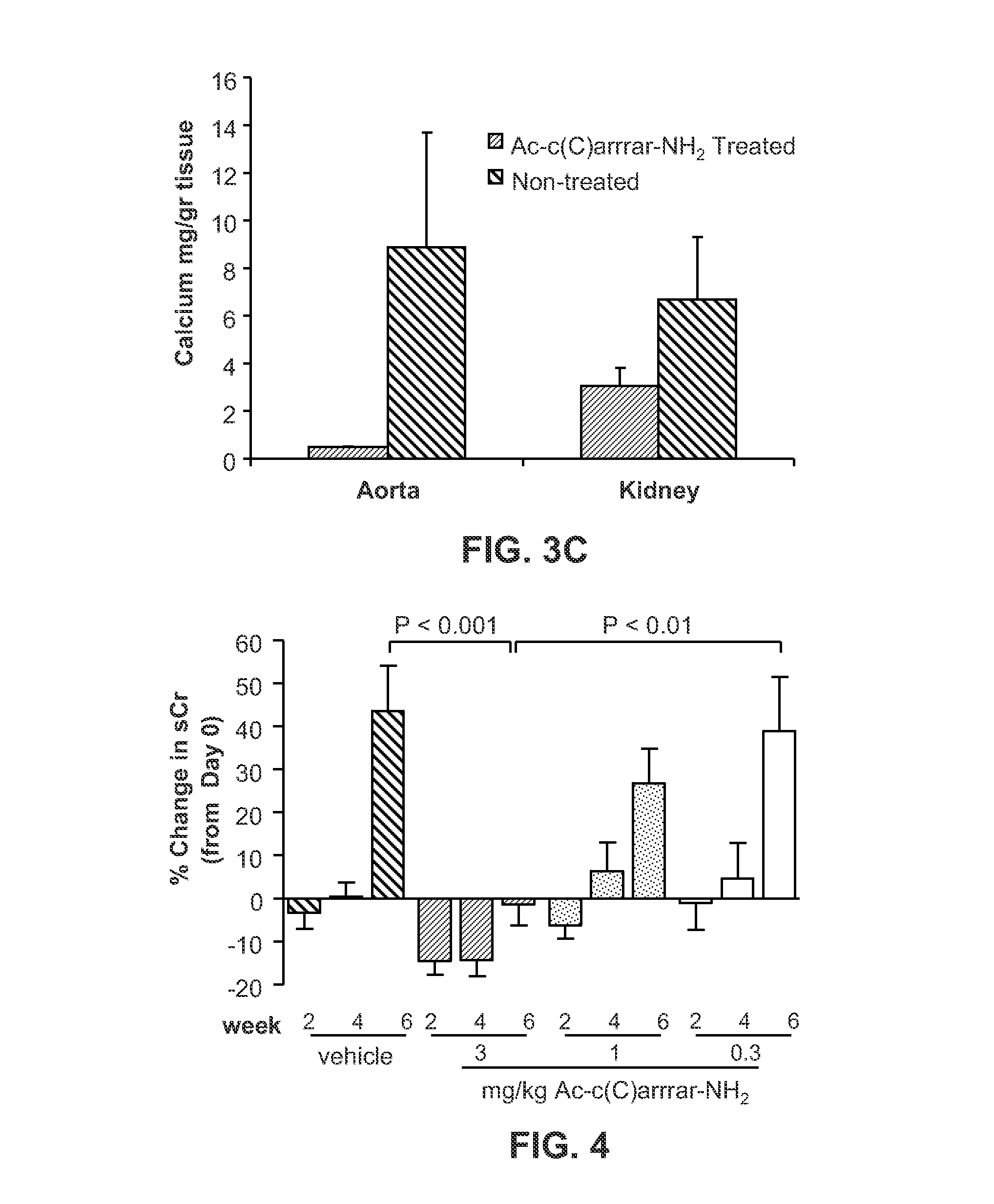
Methods for treating subjects suffering from chronic kidney disease-mineral and bone disorder or other disorders resulting in primary or secondary hyperparathyroidism are described. The methods are effective in reducing serum parathyroid hormone (PTH) levels and calcium levels in patients who undergo hemodialysis. The methods described herein are also effective in slowing the progression of kidney disease and preserving kidney function. Compositions used in the described methods are also provided and comprise calcimimetics which function as agonists of the calcium sensing receptor (CaSR).
Owner:KAI PHARMA
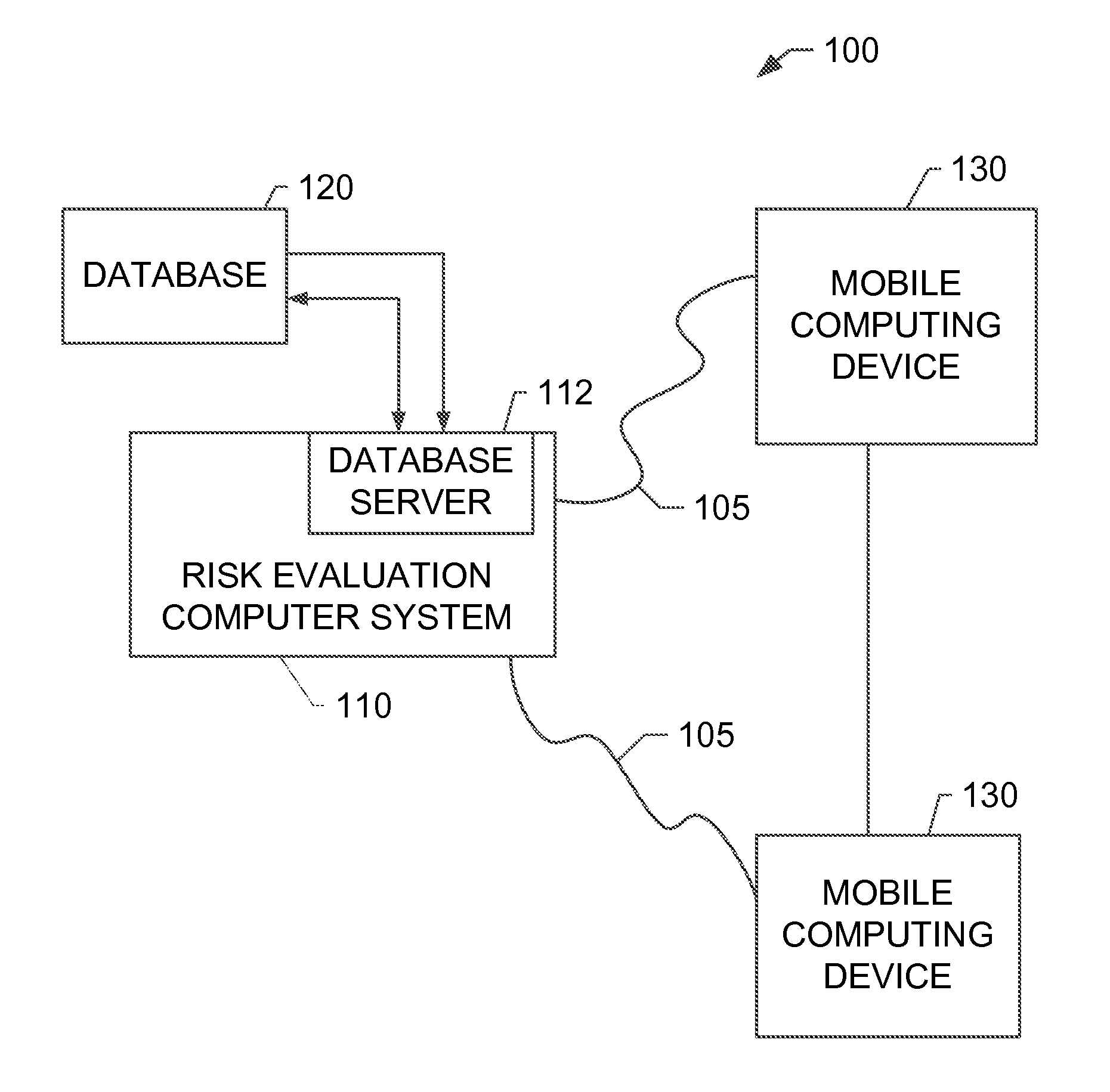
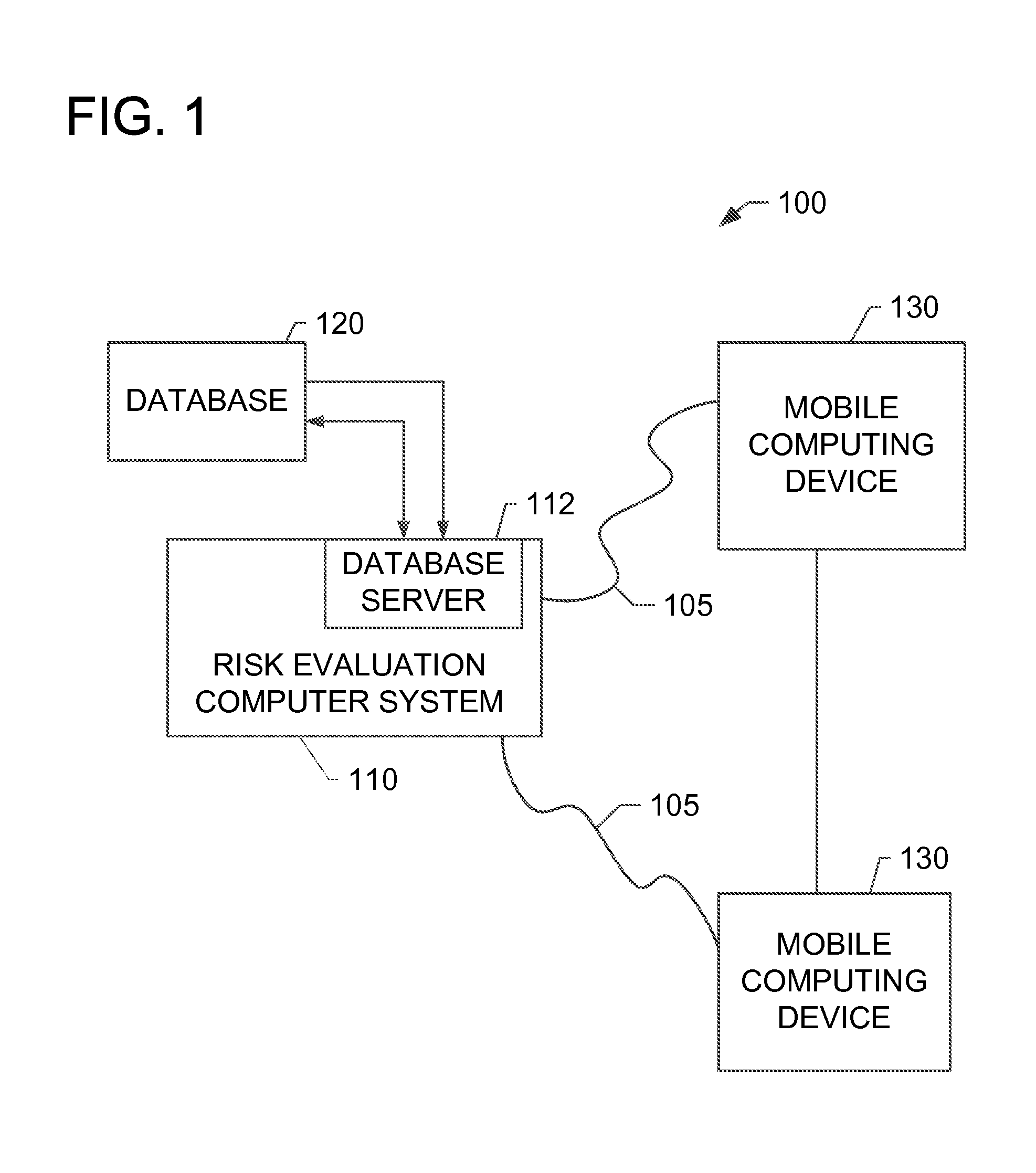
Systems and methods for determining secondary hyperparathyroidism risk factors
InactiveUS20150220698A1Medical simulationData processing applicationsFiltrationSecondary hyperparathyroidism
A computer-implemented method for determining at least one secondary hyperparathyroidism risk factor (“SHPT”) for a patient is implemented using a risk evaluation computer system in communication with a memory. The method includes receiving a plurality of demographic data associated with a patient from a mobile computing device, receiving a concentration of a renal filtration marker associated with the patient from the mobile computing device, and determining at least one SHPT risk factor for the patient based on the plurality of demographic data and the concentration of the renal filtration marker using at least one estimating equation, the SHPT risk factor indicating a likelihood that the patient has SHPT.
Owner:ABBVIE INC
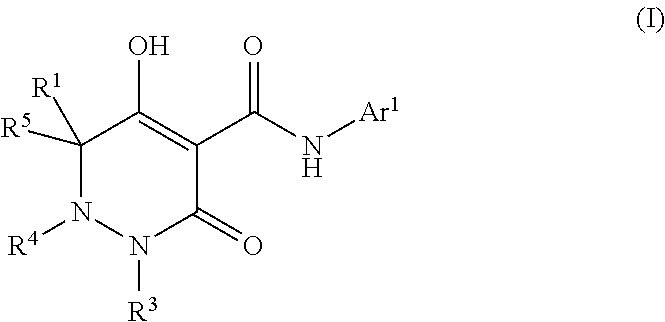
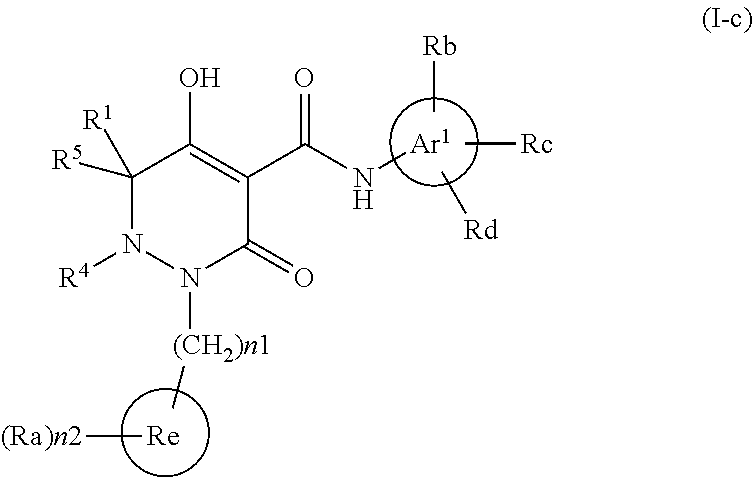
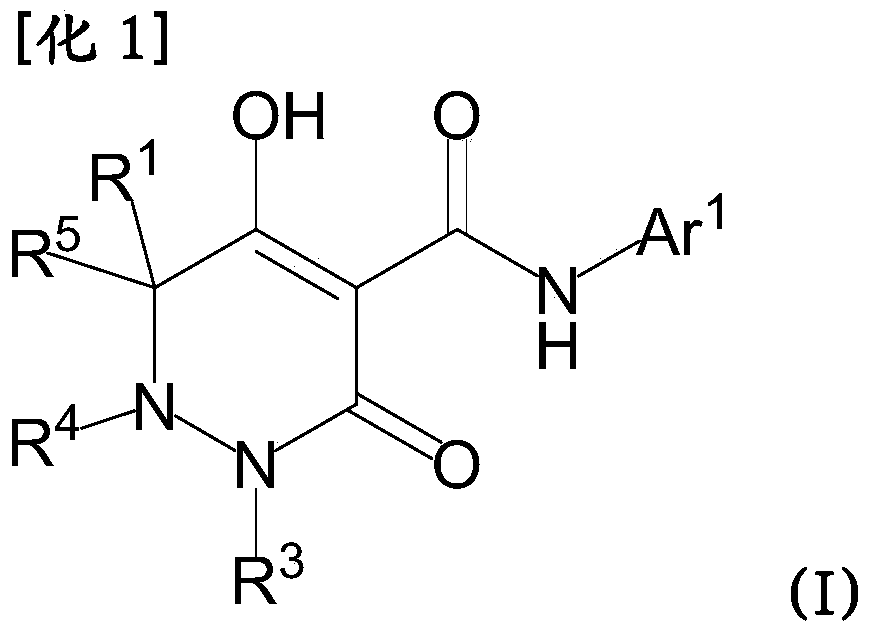
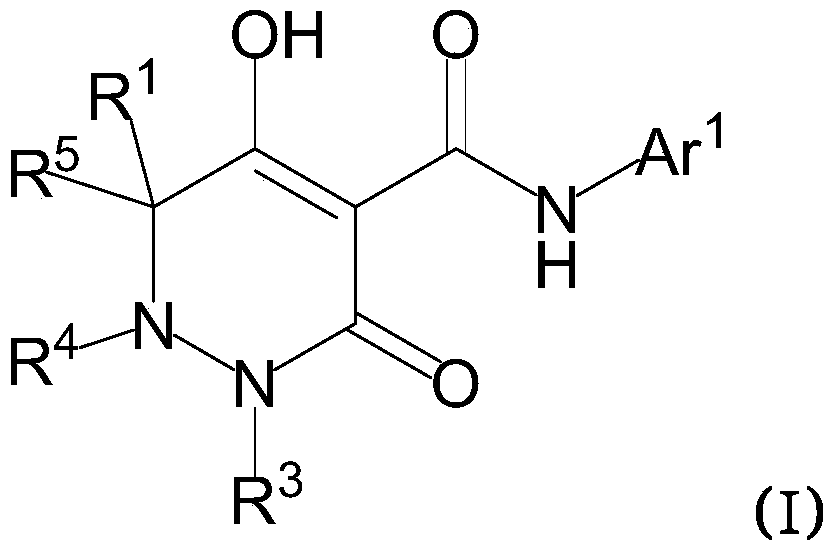
Dihydropyridazine-3,5-dione derivative and pharmaceuticals containing the same
ActiveUS20160002251A1Enhanced inhibitory effectHeavy metal active ingredientsOrganic chemistryChronic kidney failurePyridazine
The present invention provides a dihydropyridazine-3,5-dione derivative or a salt thereof, or a solvate of the compound or the salt, a pharmaceutical drug, a pharmaceutical composition, a sodium-dependent phosphate transporter inhibitor, and a preventive and / or therapeutic agent for hyperphosphatemia, secondary hyperparathyroidism, chronic renal failure, chronic kidney disease, and arteriosclerosis associated with vascular calcification comprising the compound as an active ingredient, and a method for prevention and / or treatment.
Owner:CHUGAI PHARMA CO LTD
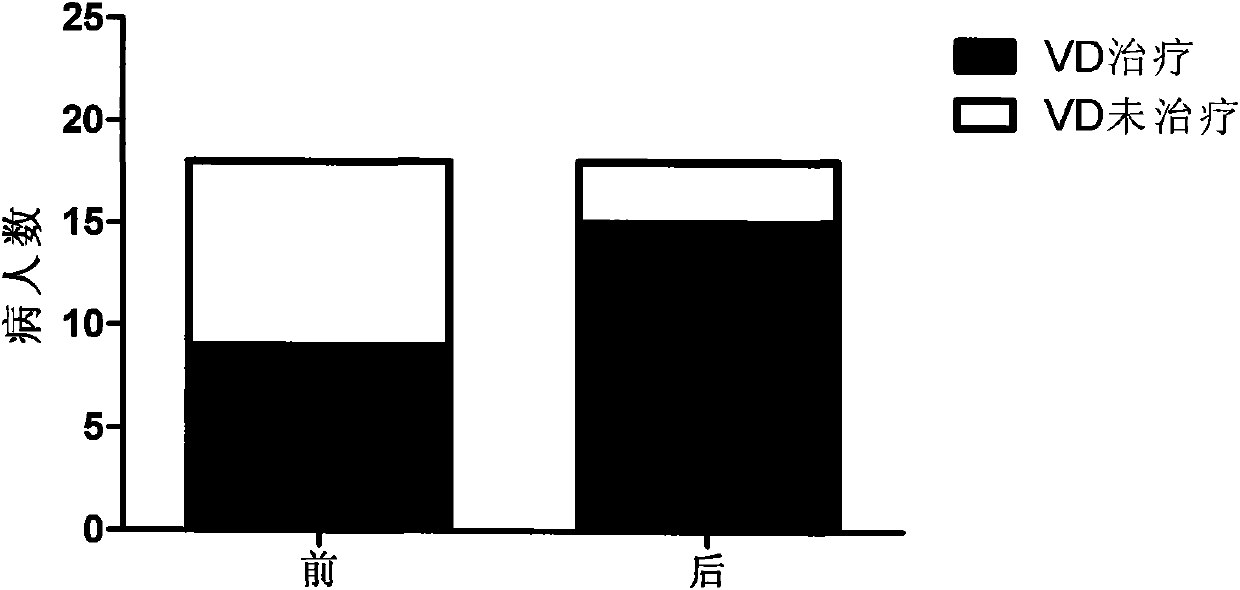
Application of medicinal carbon in preparation of medicaments for curing hyperphosphatemia
InactiveCN101904868AReduce deathReduce productionMetabolism disorderCarbon active ingredientsPeritoneal dialysisSecondary hyperparathyroidism
The invention relates to novel medicinal application of medical carbon, in particular to the application of the medicinal carbon in the preparation of medicaments for curing hyperphosphatemia. In the invention, clinical tests prove that the medicinal carbon, when taken orally, can reduce the serium inorganic phosphorus level and the product of calcium and phosphorus of patients who have received hemodialysis and peritoneal dialysis but do not have the hyperphosphatemia controlled after receiving the treatment by the calcium-containing phosphate binder, and provides a voltage detection (VD) therapy opportunity for patients with secondary hyperparathyroidism which cannot be treated by the VD therapy because of over-high product of calcium and phosphorus. The medical carbon is favorable for reducing and preventing angiocardiopathy development death, caused by chronic kidney disease-mineral and bone disorder (CKD-MBD), of the dialysis patients. Thus, the medicinal carbon can be used as novel medicaments for curing the hyperphosphatemia of patients with dialysis.
Owner:河北长天药业有限公司
19-nor-vitamin D analogs with 3,2-dihydrofuran ring
19-nor-vitamin D analogs having an additional dihydrofuran ring connecting the 3β-oxygen and carbon-2 of the A-ring of the analog, and pharmaceutical uses therefore, are described. These compounds exhibit selective in vitro and in vivo activities, making them therapeutic agents for the treatment or prophylaxis of autoimmune diseases, some types of cancers, metabolic bone diseases, osteomalacia, osteopenia, secondary hyperparathyroidism, psoriasis, or other skin diseases.
Owner:WISCONSIN ALUMNI RES FOUND
Treatment of chronic kidney disease (CKD) subjects using lanthanum compounds
InactiveUS20070104799A1Heavy metal active ingredientsBiocideSecondary hyperparathyroidismCalcification
Owner:SHIRE INT LICENSING
Oral solution containing calcitriol and preparation method thereof
ActiveCN114831933AMeet clinical needsSpeed up replenishmentOrganic active ingredientsFilling using counterpressureHydroxybenzoate EthersSecondary hyperparathyroidism
The invention relates to the technical field of calcitriol, in particular to an oral solution containing calcitriol and a preparation method of the oral solution, and the oral solution containing calcitriol comprises the following components in parts by weight: 0.290-0.305 part of calcitriol; 29.7 to 30.3 parts of butyl hydroxy anisole; 29.8 to 30.2 parts of butylated hydroxytoluene (BHM); and 299987 to 300012 parts of medium chain triglycerides. The preparation method of the oral solution containing calcitriol comprises the following steps: S100, weighing: turning on a yellow light lamp in a weighing room, weighing calcitriol, medium chain triglyceride, butylated hydroxyanisole and butylated hydroxytoluene according to parts by weight, and carrying out double-person rechecking; compared with the prior art, the oral solution containing calcitriol has the following beneficial effects that the oral solution containing calcitriol can make up the blank in the field of treating secondary hyperparathyroidism of patients with moderate to severe chronic renal failure before dialysis and follow-up metabolic bone diseases and hypoparathyroidism in domestic children medication, and the clinical requirements are met.
Owner:CP PHARMA QINGDAO CO LTD
Methods of Treating Osteoporosis and Secondary Hyperparathyroidism Using 20-Methyl, Gemini Vitamin D3 Compounds
InactiveUS20090298799A1Strong therapeutic activityReduce adverse side effectsOrganic active ingredientsBiocideSecondary hyperparathyroidismOsteopetrosis
The invention provides for methods of using 20-methyl Gemini vitamin D3 compounds to treat osteoporosis and secondary hyperparathyroidism.
Owner:GALAPAGOS SASU +1
19-nor-vitamin d analogs with 3,2-dihydrofuran ring
19-nor-vitamin D analogs having an additional dihydrofuran ring connecting the 3β-oxygen and carbon-2 of the A-ring of the analog, and pharmaceutical uses therefore, are described. These compounds exhibit selective in vitro and in vivo activities, making them therapeutic agents for the treatment or prophylaxis of autoimmune diseases, some types of cancers, metabolic bone diseases, osteomalacia, osteopenia, secondary hyperparathyroidism, psoriasis, or other skin diseases.
Owner:WISCONSIN ALUMNI RES FOUND
19-nor-vitamin D analogs with 1,2-dihydrofuran ring
Owner:WISCONSIN ALUMNI RES FOUND
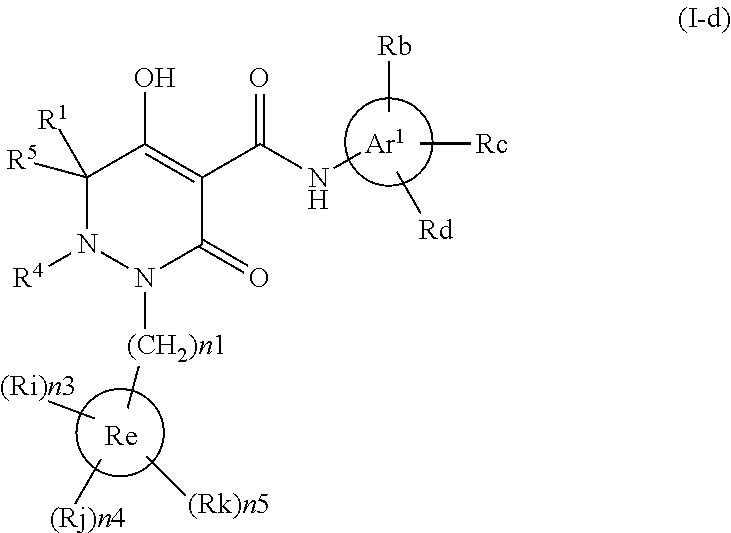
Dihydropyridazine-3,5-dione derivative
ActiveCN105073715AExcellent NaPi-IIb inhibitionExcellent PiT-1 inhibitionOrganic chemistryMetabolism disorderSodium dependentSecondary hyperparathyroidism
Owner:CHUGAI PHARMA CO LTD
Methods and compositions for reducing parathyroid levels
InactiveCN105796530AOrganic active ingredientsPeptide/protein ingredientsSerum igeSecondary hyperparathyroidism
Methods and compositions for reducing serum parathyroid levels in, for example, chronic kidney disease patients are disclosed. In these methods, an effective amount of a modified release formulation of 25 -hydroxy vitamin D is orally administered to a patient suffering from secondary hyperparathyroidism to lower the patient' s serum intact parathyroid hormone (iPTH) level, while avoiding a surge in serum total 25-hydroxy vitamin D.
Owner:CYCTOCHROMA INC +1
Use of 2-methylene-19-nor-(20S)-1-alpha,25-dihydroxyvitamin D3 to treat secondary hyperparathyroidism in patients previously treated with calcimimetics
InactiveUS9539264B2Prevent secondary hyperparathyroidismReduce and eliminate needOrganic active ingredientsPharmaceutical delivery mechanismMedicinePreviously treated
Disclosed are methods of administering 2-methylene-19-nor-(20S)-1α,25-dihydroxyvitamin D3 to treat and / or prevent secondary hyperparathyroidism and / or its accompanying symptoms in a subject having or at risk for developing secondary hyperparathyroidism, including a subject previously administered a calcimimetic.
Owner:WISCONSIN ALUMNI RES FOUND
Preparation method of paricalcitol
InactiveCN103896817ARaw materials are cheap and easy to getShort process stepsOrganic chemistryWittig reactionNandrolone
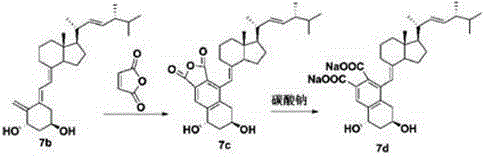
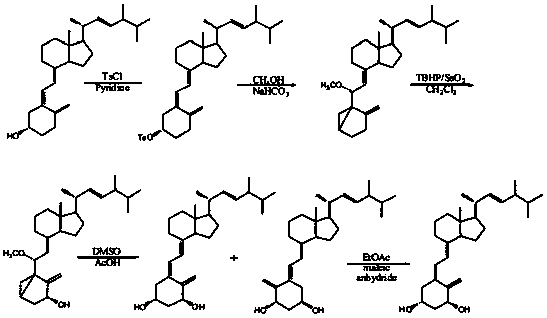
The invention discloses a method for preparing paricalcitol. Nandrolone is taken as a raw material, oxidation, Wittig reaction, hydroxyl protection, bromination, rearrangement, hydrogen bromide removal, addition, oxidation, reduction, hydroxyl deprotection and ring opening are carried out, so that the paricalcitol is obtained. The paricalcitol is used for preventing and treating secondary hyperparathyroidism.
Owner:湖南华腾制药有限公司
Adjunctive therapy with 25-hydroxyvitamin D
InactiveCN106604733AOrganic active ingredientsPeptide/protein ingredientsSecondary hyperparathyroidismCvd risk
Methods, compositions, and kits for adjunctive therapy using 25-hydroxyvitamin D are disclosed. The 25-hydroxyvitamin D may be administered with an agent that increases the risk of hypocalcemia and / or an anticancer agent. The adjunctive therapy is effective to treat and prevent iatrogenic hypocalcemia and / or secondary hyperparathyroidism, as well as delay cancer progression and the time to a post-treatment skeletal related event.
Owner:OPKO IRELAND GLOBAL HLDG LTD
Methods and compositions for reducing parathyroid levels
InactiveCN103037902AOrganic active ingredientsPeptide/protein ingredientsSerum igeSecondary hyperparathyroidism
Methods and compositions for reducing serum parathyroid levels in, for example, chronic kidney disease patients are disclosed. In these methods, an effective amount of a modified release formulation of 25 -hydroxy vitamin D is orally administered to a patient suffering from secondary hyperparathyroidism to lower the patient' s serum intact parathyroid hormone (iPTH) level, while avoiding a surge in serum total 25 -hydroxy vitamin D.
Owner:CYCTOCHROMA INC +1
Capsule formulations containing lanthanum compounds
InactiveUS20120141580A1Powder deliveryHeavy metal active ingredientsSecondary hyperparathyroidismHyperphosphoremia
The present invention includes an oral pharmaceutical capsule comprising a shell, lanthanum carbonate or lanthanum carbonate hydrate, and a lubricant such as talc, wherein the shell encapsulates the lanthanum carbonate or lanthanum hydrate and the lubricant. Capsule shells comprise, for example, gelatin. The capsules of the present invention dissolve at a similar rate before and after storage. The oral pharmaceutical capsules of the present invention can be administered to treat a patient at risk for or suffering from hyperphosphatemia, at risk for or suffering from chronic kidney disease (CKD), at risk for or suffering from soft tissue calcification associated with CKD, or at risk for or suffering from secondary hyperparathyroidism.
Owner:SHIRE PLC
Adjunctive therapy with 25-hydroxyvitamin D and articles therefor
InactiveCN108135868AOrganic active ingredientsPeptide/protein ingredientsCinacalcetSkeletal related events
Methods, compositions, and kits for adjunctive therapy using 25-hydroxyvitamin D are disclosed. The 25-hydroxyvitamin D may be administered with an agent that increases the risk of hypocalcemia, suchas cinacalcet or a pharmaceutically acceptable salt thereof, and / or an anticancer agent. The adjunctive therapy is effective to treat and prevent iatrogenic hypocalcemia and / or secondary hyperparathyroidism, as well as delay cancer progression and the time to a post-treatment skeletal related event.
Owner:OPKO IRELAND GLOBAL HLDG LTD
A new crystalline form of docalciferol and its preparation method
ActiveCN105237452BGood physical and chemical propertiesImprove stabilityOrganic active ingredientsOrganic chemistryDiseaseCalciferols
The invention belongs to the field of medicinal chemistry, and in particular relates to a new crystal form A of docalciferol and a preparation method thereof. The invention also relates to a pharmaceutical composition containing the new crystal form A of docalciferol and its role in the treatment of osteoporosis Hyperparathyroidism or secondary hyperparathyroidism. The new crystal form A of docalciferol, using Cu-Kα radiation, X-ray powder diffraction at 2θ angles at 7.82, 8.51, 12.06, 13.01, 15.60, 17.05, 17.82, 18.71, 19.35, 20.06, There are diffraction peaks at 20.37, 20.62, 21.98, 22.68, 22.90, 23.81, and 24.79. At the same time, the invention also discloses a process improvement scheme for preparing high-purity doxercalciferol, which has simple steps, easy operation, short time consumption and low cost, and is very beneficial to large-scale industrial production.
Owner:NANJING HERON PHARM CO LTD
Capsule and powder formulations containing lanthanum compounds
The present invention includes an oral pharmaceutical capsule comprising a shell, lanthanum carbonate or lanthanum carbonate hydrate, and a lubricant such as talc, wherein the shell encapsulates the lanthanum carbonate or its hydrate and the lubricant. Capsule shells comprise, for example, gelatin. The present invention also includes an oral pharmaceutical powder comprising lanthanum carbonate or lanthanum carbonate hydrate and a pharmaceutically acceptable excipient. The oral pharmaceutical capsules and powders of the present invention can be administered to treat a patient at risk of or suffering from hyperphosphatemia, at risk of or suffering from chronic kidney disease (CKD), at risk of or suffering from soft tissue calcification associated with CKD, or at risk of or suffering from secondary hyperparathyroidism.
Owner:TAKEDA PHARMA CO LTD
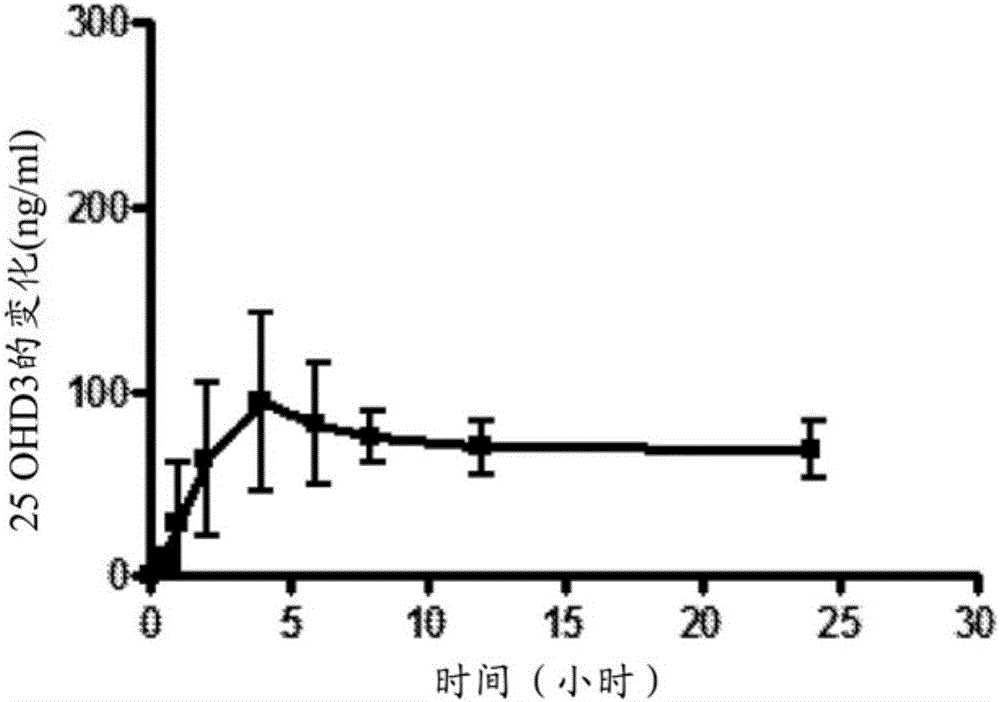
Methods of vitamin d treatment
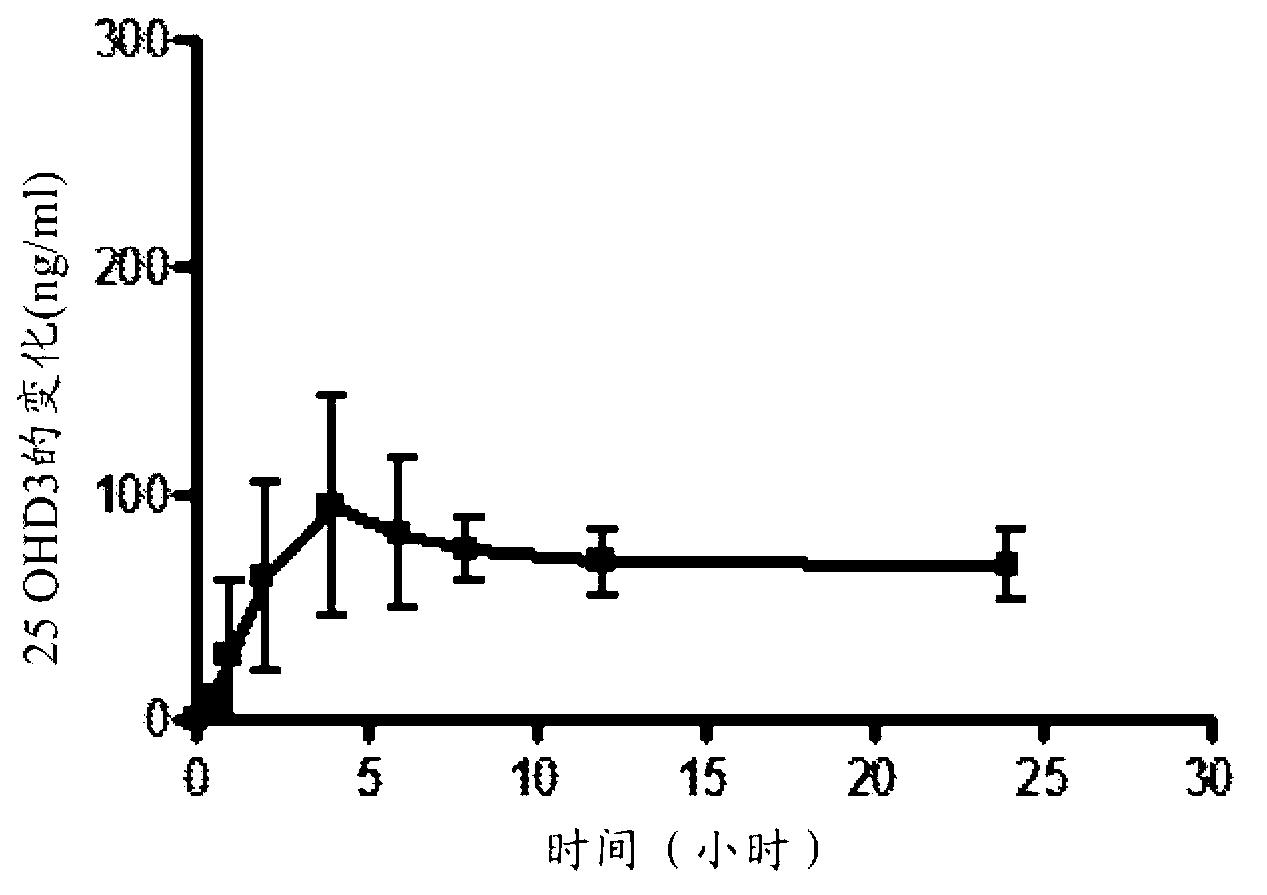
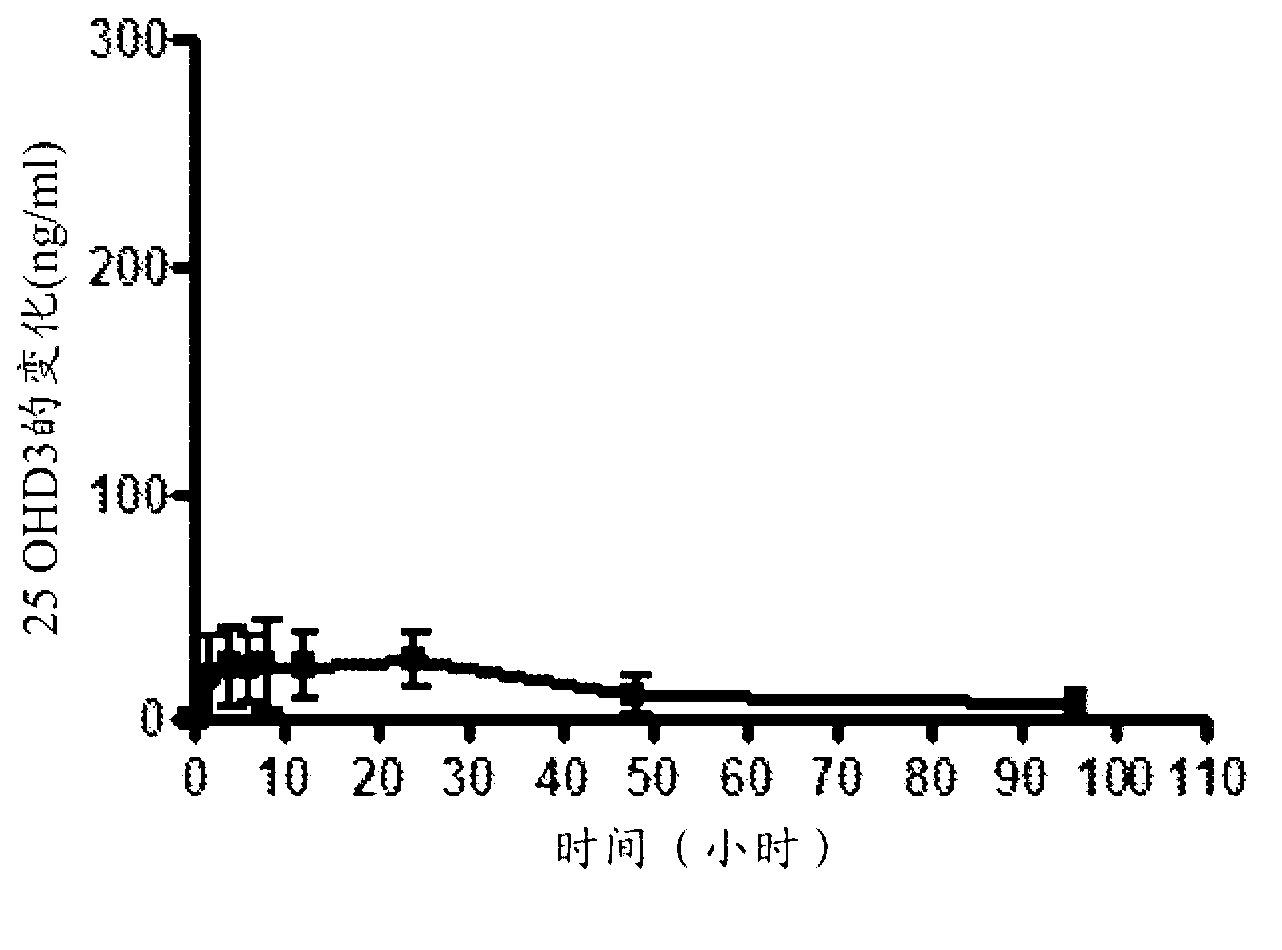
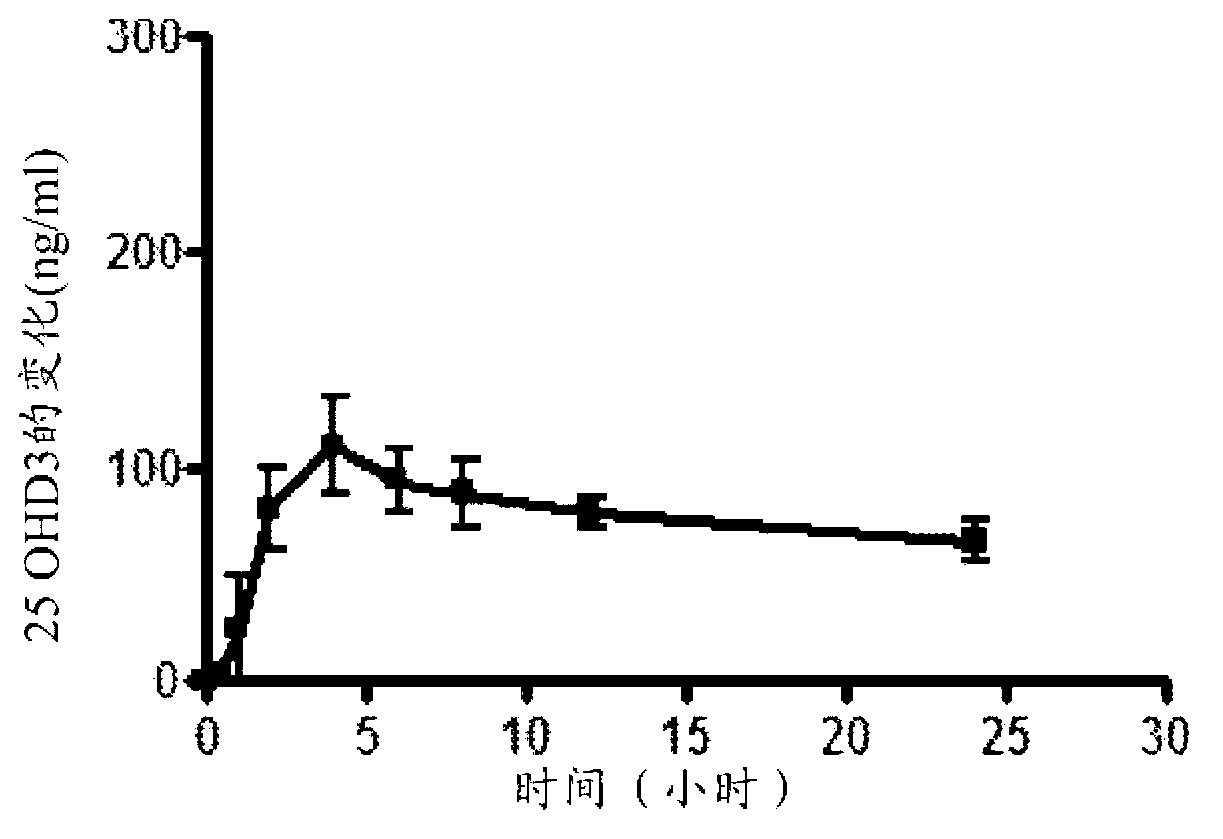
Methods for treating vitamin D insufficiency and secondary hyperparathyroidism in patients having CKD comprising administering repeat doses of 25-hydroxyvitamin D are disclosed. The methods comprise administering 25-hydroxyvitamin D in an amount effective to safely raise the patient's serum 25-hydroxyvitamin D level to greater than 90 ng / ml and / or to control the patient's serum ratio of 25-hydroxyvitamin D to 24,25-dihydroxyvitamin D to less than 20.
Owner:OPKO IRELAND GLOBAL HLDG LTD
Rhythmic photostimulation pattern system and application thereof
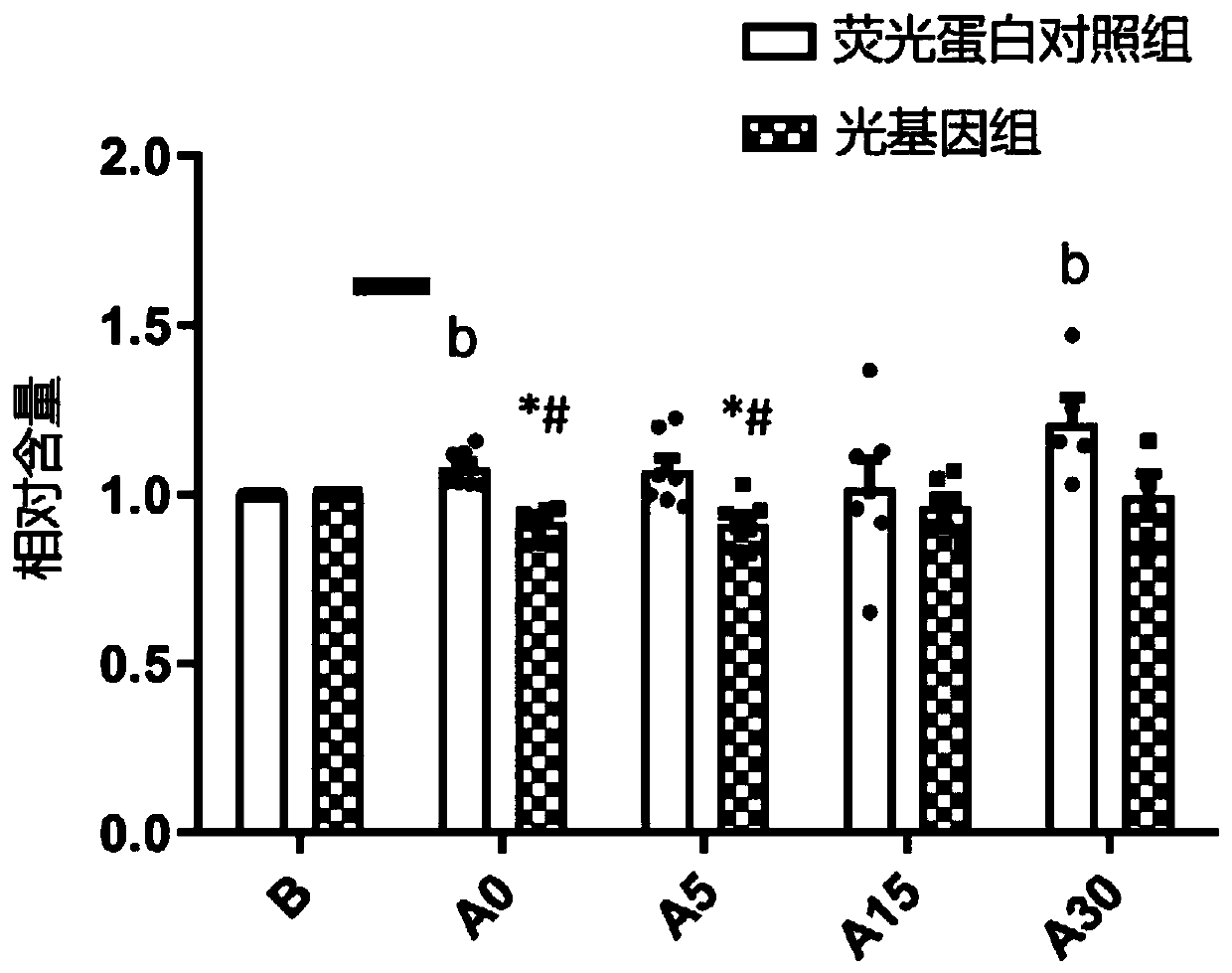
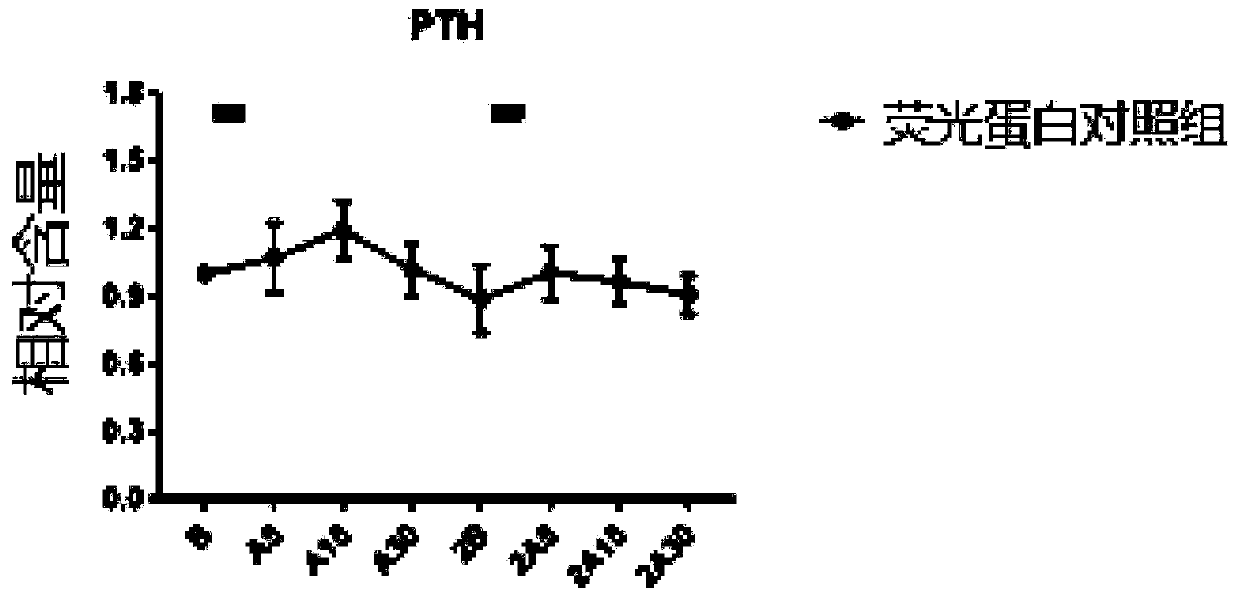
The invention relates to a rhythmic photostimulation pattern system and application thereof. In the rhythmic photostimulation pattern system, a cycle of photostimulation is four weeks, the frequency of photostimulation is three times per week, and the time of photostimulation at a time is 10 to 40 minutes. The rhythmic photostimulation pattern system disclosed by the invention can be used for effectively improving osteoporosis induced by secondary hyperparathyroidism, remarkably inhibiting excessive secretion of parathyroid hormones induced by the secondary hyperparathyroidism, remarkably reversing bone density and / or bone bulk density decrease induced by the secondary hyperparathyroidism, activating differentiating and aging of osteoblasts and inhibiting differentiating and aging of osteoclasts, and thus, osteogenesis can be greater than osteoclastogenesis. The rhythmic photostimulation pattern system is very convenient in operating mode, and an entire process is free of introductionof drug for intervention, so that a side effect resulting from employing the system is lower compared with that of drug therapy, and the safety is higher.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
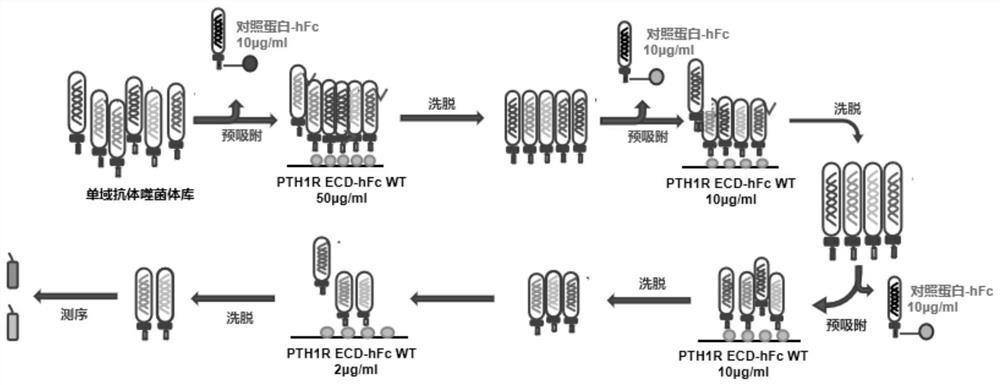
Anti-human PTH1R extracellular region blocking type single-domain antibody and application thereof
ActiveCN114874325AReduce functionInhibit normal functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesSecondary hyperparathyroidism
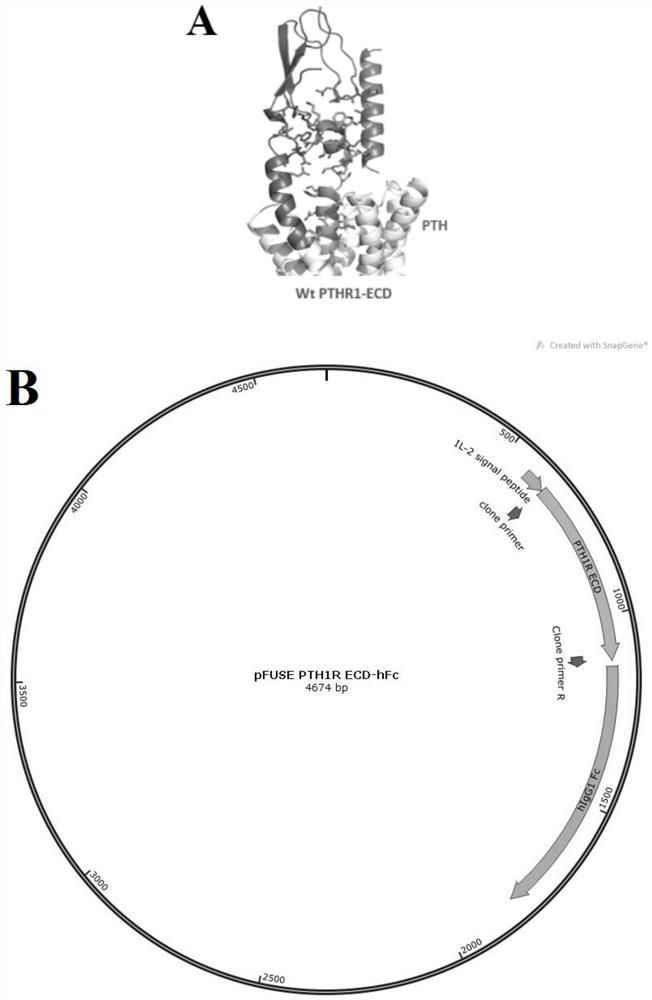
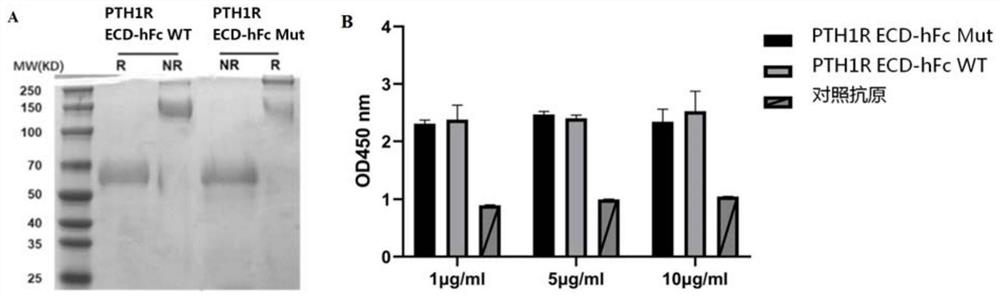
The invention discloses a fully humanized monoclonal single-domain antibody for resisting human PTH1R and an application of the fully humanized monoclonal single-domain antibody. The amino acid sequence of the antibody is shown as SEQ ID NO: 2. According to the invention, aiming at wild and mutant human PTH1R ECD proteins, antibody screening is carried out by using a phage display technology, and the anti-human PTH1R blocking type single-domain antibody with a blocking effect is screened and obtained through methods such as ELISA, FACS and the like. The antibody can be combined with human PTH1R, can block the interaction between PTH and PTH1R, and has a potential application value in human body dysfunction mediated by PTH-PTH1R, such as chronic kidney disease secondary hyperparathyroidism.
Owner:NANJING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com