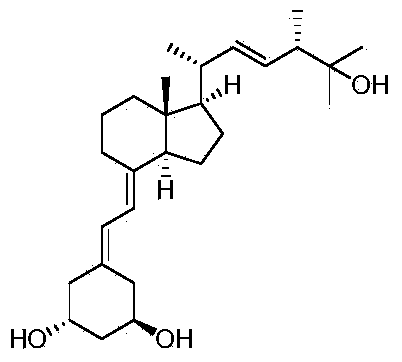
Preparation method of paricalcitol
A technology of paricalcitol and its compounds, which is applied in the field of new synthesis of chemical drugs, and can solve problems such as the lack of application experience in patients with hepatic insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
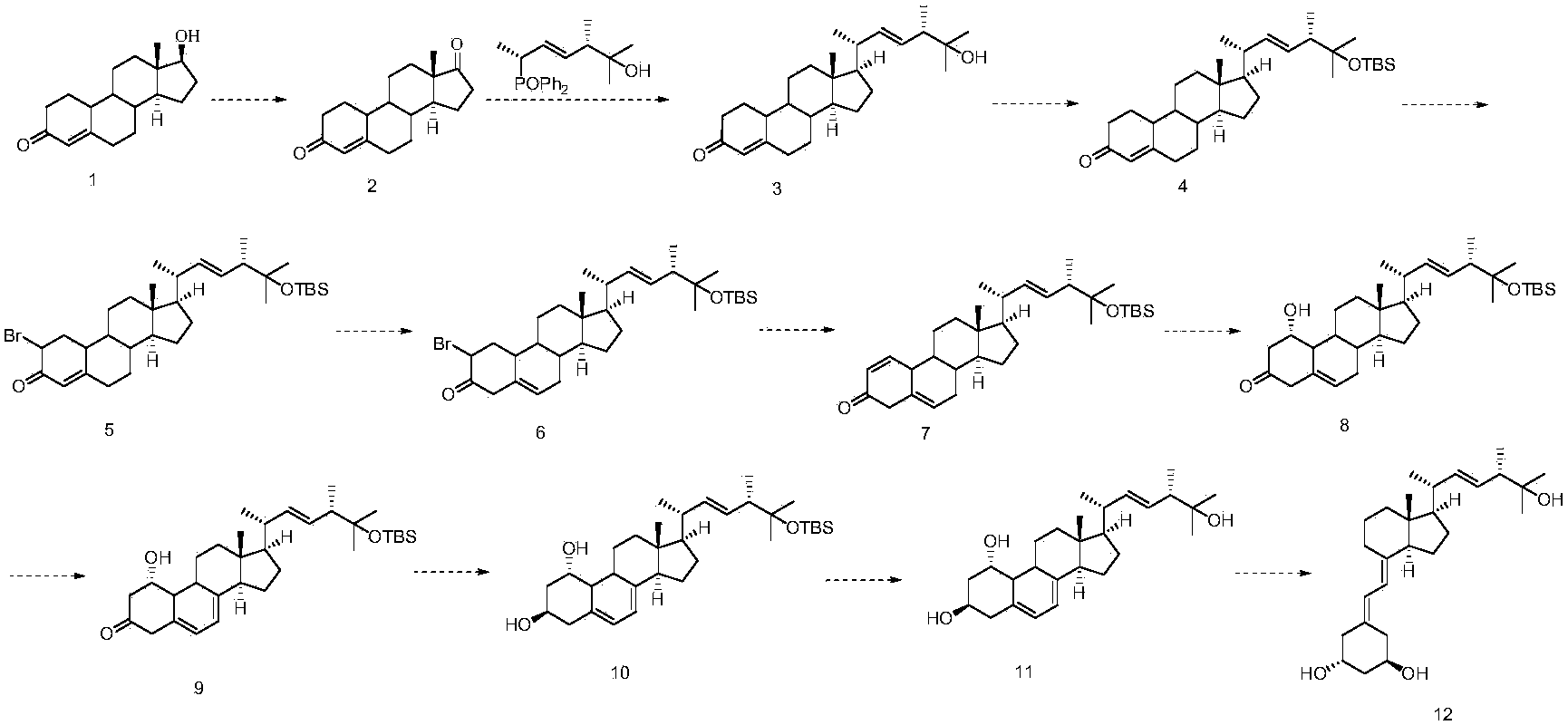
Embodiment 1
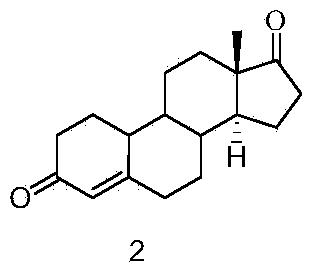
[0036] (1) Synthesis of compound 2
[0037] 60 g of Nandrolone and 45 g of pyridinium chlorochromate were added to 400 ml of dichloromethane, stirred at room temperature, filtered, the mother liquor was concentrated under reduced pressure, and the residue was separated by a chromatographic column to obtain 49 g of the product.
[0038] (2) Synthesis of compound 3
[0039] 49g of compound 2 and 21g of (3S,6R,E)-6-(diphenylphosphine)-2,3-dimethylhept-4-en-2-ol were added to 350ml of tetrahydrofuran, cooled to - 78oC, slowly add 14g of sodium amide, stir for 5 hours, naturally rise to room temperature, add saturated ammonium chloride, then add ethyl acetate for extraction, collect the organic phase, concentrate, and the residue is separated by a chromatographic column to obtain 52g of product.
[0040] (3) Synthesis of compound 4
[0041] Add 52g of compound 3 to 300ml of dichloromethane, cool to 0°C, slowly add 35g of TBSCl, stir for 3 hours, add ice water, then add ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com