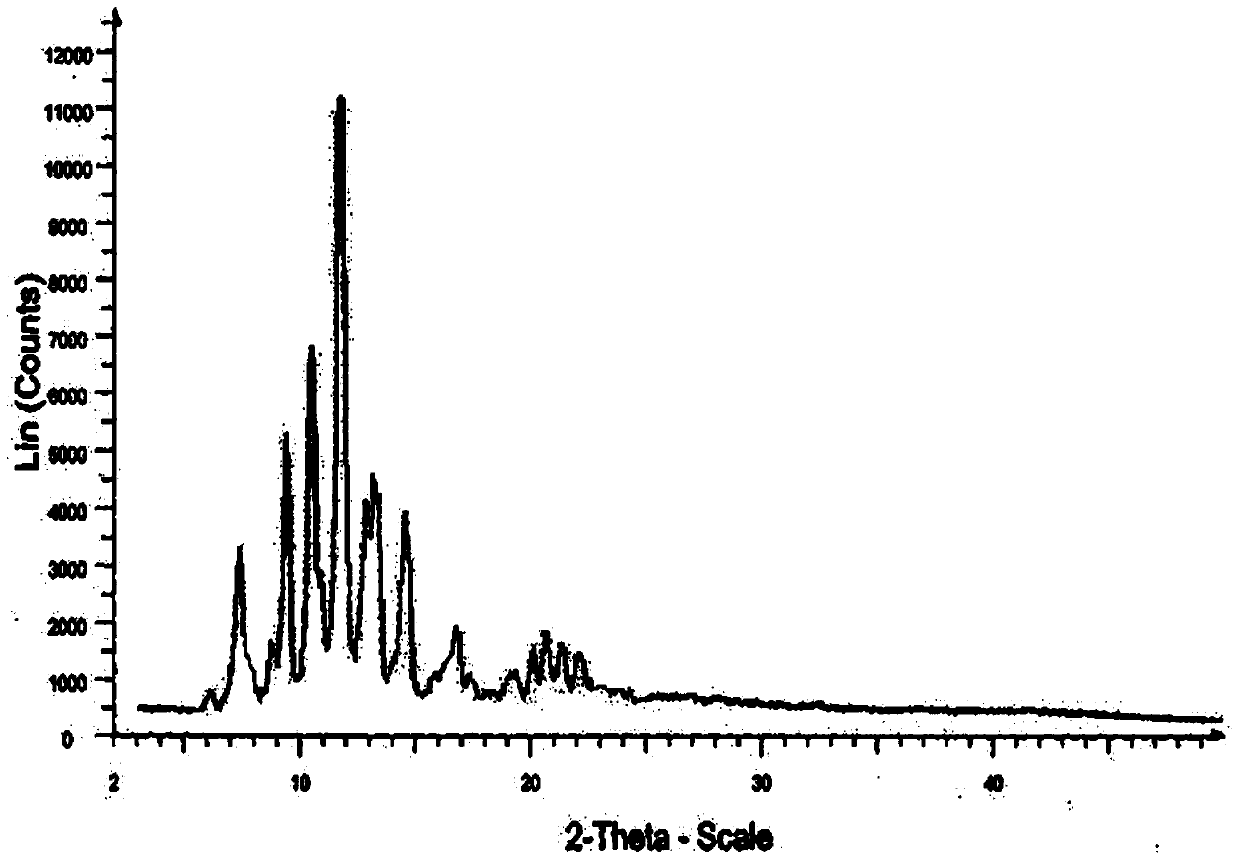
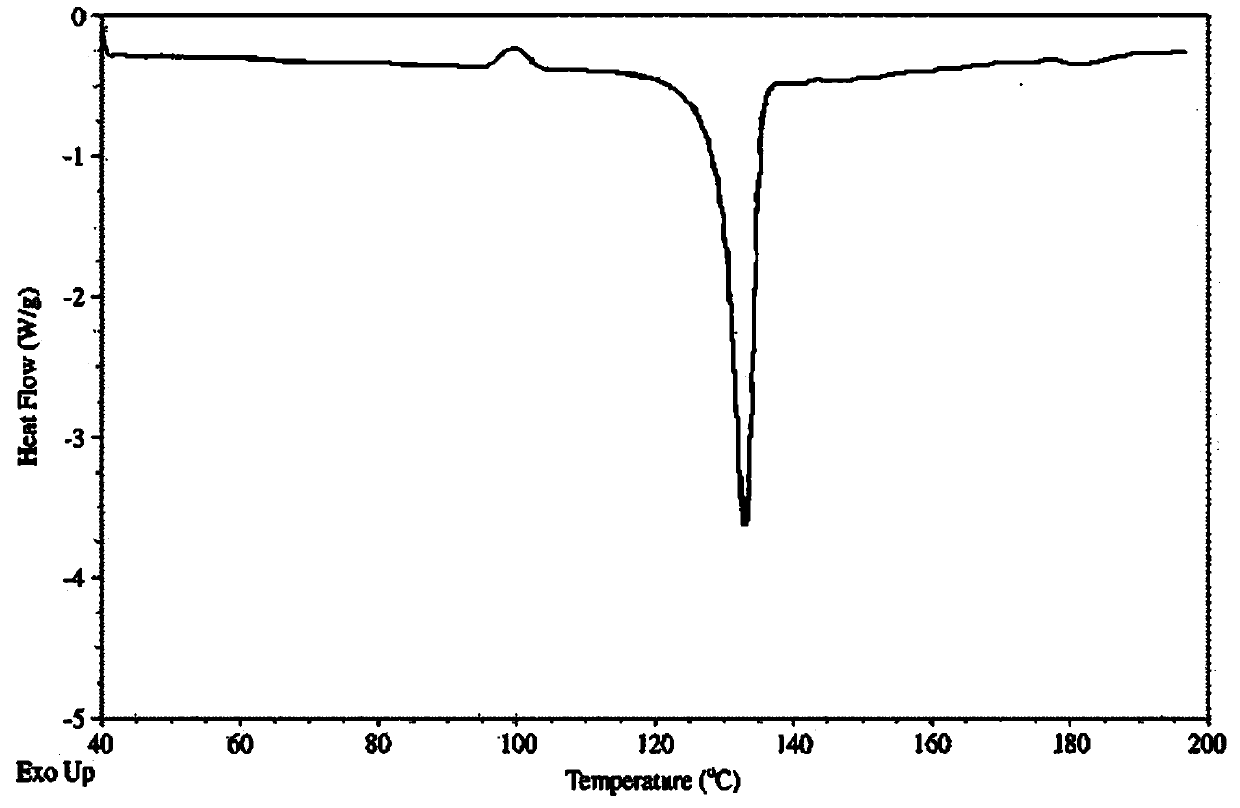
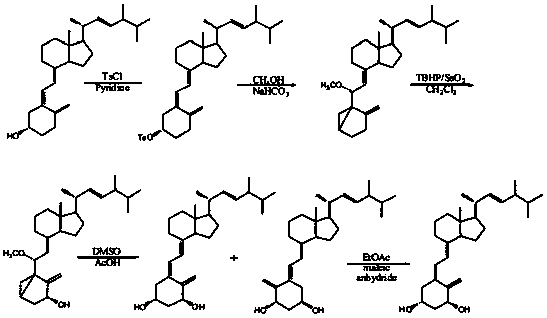
A new crystalline form of docalciferol and its preparation method
A technology of docalciferol and crystal form, which is applied in the new crystal form of docalciferol and its preparation field, can solve the problems of poor stereoselectivity, low total yield of the route, and difficulty in separation and purification of the final product, and achieve easy operation , Good physical and chemical properties, conducive to the effect of large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of new crystal form A of degree-calcidol
[0053] (1) Put vitamin D 2 (The compound of formula 2) Dissolve about 15g in 100mL of dichloromethane, cool to -15 to -20°C, and slowly pass SO through a steel bottle 2 Gas, keep the reaction for 1-2h, TLC monitors the reaction to be complete, rotary evaporation to remove SO 2 Gas to obtain a foamy yellow solid, 18.3 g of the compound of formula 3;
[0054] (2) Add 18.3 g of the compound of formula 3 to about 130 mL of DMF, 11 g of imidazole, 13 g of tert-butyldimethylchlorosilane, and stir at room temperature for 2 hours. TLC monitors that the reaction is complete. Add 300 mL of water and extract with ethyl acetate (3*100 mL) , Combine the organic phases, wash the organic phase three times with 400 mL of saturated brine, dry and concentrate the organic phase to obtain a yellow oil, that is, about 22.1 g of the compound of formula 4;
[0055] (3) Dissolve about 22.1g of the compound of formula 4 in a mixture of 2...
Embodiment 2
[0065] Example 2 Preparation of new crystal form A of calcidiol
[0066] (1) Dissolve about 18g of vitamin D2 (compound of formula 2) in 120mL of dichloromethane, cool to -15 to -20°C, slowly inject sulfur dioxide gas through a steel bottle, keep the reaction for 1-2h, and TLC monitor the reaction to be complete , Rotary evaporation to remove sulfur dioxide to obtain a foamy yellow solid, 21.3g of the compound of formula 3;
[0067] (2) Add 21.3g of compound of formula 3, add about 150mL DMF, 13g imidazole, 15g tert-butyldimethylchlorosilane, stir at room temperature for 2h, TLC monitor the reaction is complete, add 300mL water, and extract with ethyl acetate (3*100mL) , Combine the organic phases, wash the organic phase three times with 400 mL of saturated brine, dry and concentrate the organic phase to obtain a yellow oil, that is, about 24.1 g of the compound of formula 4;
[0068] (3) Dissolve about 24.1g of the compound of formula 4 in a mixture of 250mL ethanol and 15mL water,...
Embodiment 3
[0078] Example 3 Preparation of new crystal form A of calcidiol
[0079] The preparation of high-purity docalcidol is the same as in Example 1, and the HPLC purity is 99.2%
[0080] Crystal preparation process:
[0081] a) At a temperature of 35-40℃, stir and dissolve 6.0g of high-purity docalciferol in a mixed solvent of butanone and ethanol; among them, butanone is 6 times the amount of docalciferol, about 36mL, and ethanol is 2 times the amount of Ducalcidol is about 12mL;
[0082] b) Filter while it is hot, cool the filtrate to 10-15°C, stir slowly and crystallize for 5-10 hours;
[0083] c) The precipitated crystals are filtered and dried in a vacuum at 25-30°C to obtain about 4.7 g of new crystal form A of calcidiol, with a purity of 99.7% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com