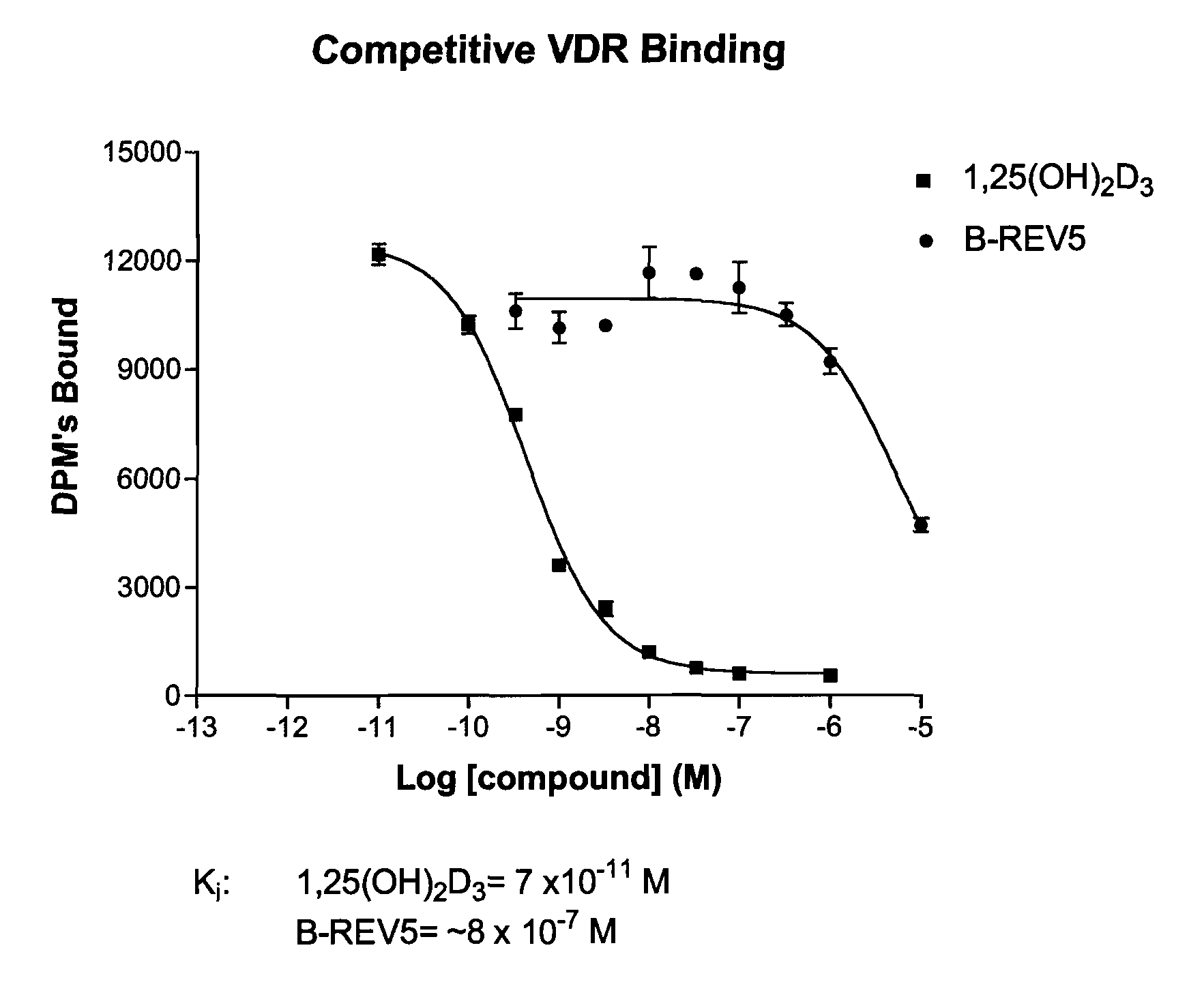
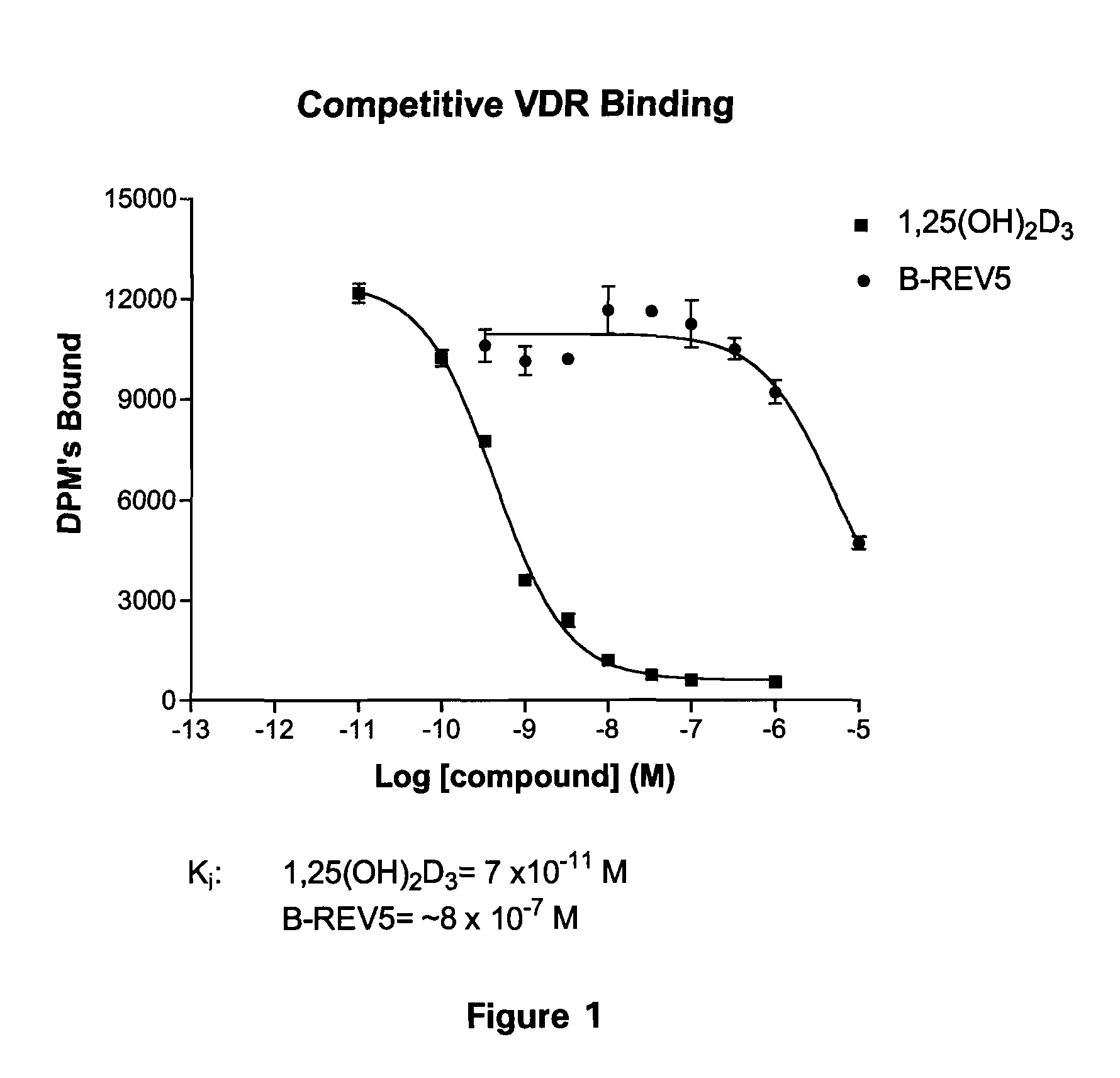
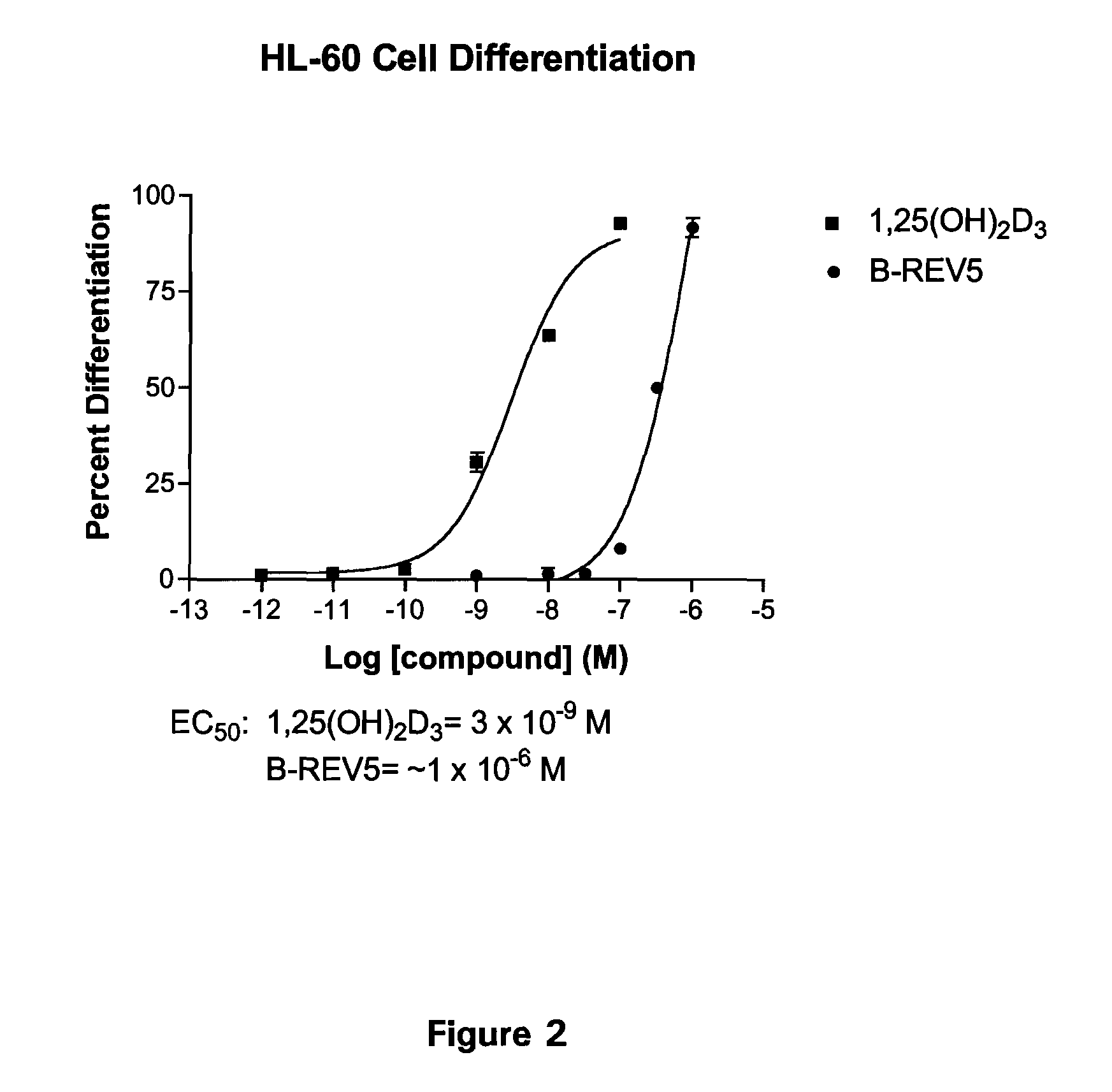
19-nor-vitamin d analogs with 3,2-dihydrofuran ring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 19-norvitamin D3 analogues 10 and 11
[0032]Referring to SCHEME I the starting cyclohexanedione 1 was obtained from commercial (−)-quinic acid according to the described procedure, Sicinski et al., J. Med. Chem. 45, 3366 (2002).
[0033](a) Selective Protection of Carbonyl Group in Diketone 1
[0034](2R,6R)-2,6-Bis[(tert-butyldimethylsilyl)oxy]-4,4-ethylenedithio-cyclohexanone (2). To a stirred solution of 1,2-ethanedithiol (0.25 mL, 3.0 mmol) and Zn(OTf)2 (646 mg, 1.78 mmol) in anhydrous methylene chloride (7.2 mL) was transferred a solution of 1 (964 mg, 2.24 mmol) in anhydrous methylene chloride (9.6 mL) at 0° C. under argon. The mixture was stirred at 0° C. for 1 h, and at room temperature for 1.5 h and then it was poured into brine and extracted with ethyl acetate. The extract was washed with saturated NaHCO3, water, 5% HCl, again water, dried (MgSO4) and evaporated. The residue was purified by column chromatography on silica. Elution with hexane / ethyl acetate (96:4) a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| NMR | aaaaa | aaaaa |
| conformational | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com