Anti-human PTH1R extracellular region blocking type single-domain antibody and application thereof
A single-domain antibody, anti-human technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Antigen Preparation
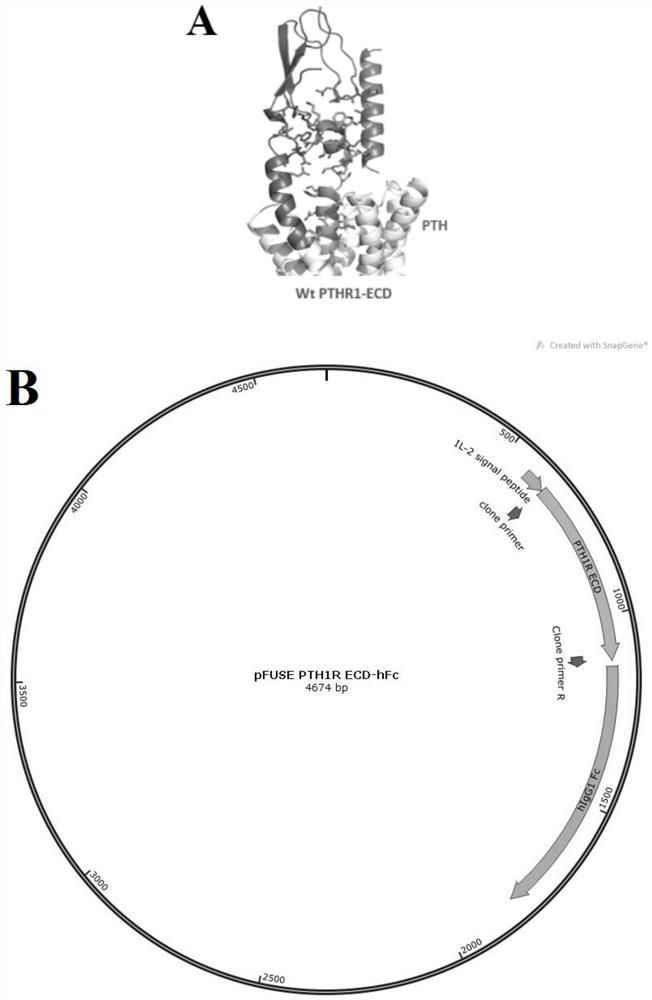
[0033] 1.1 Construction and purification of wild-type and mutant human PTH1R ECD-hFc plasmids
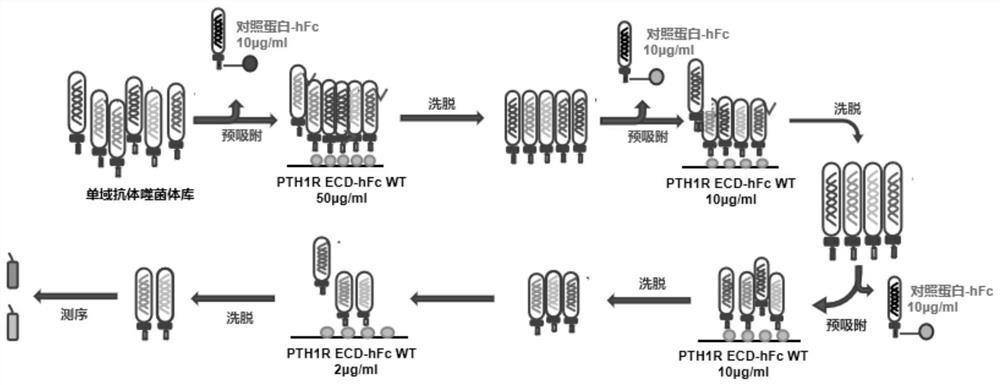
[0034] According to the structure of human PTH1R ECD, the key site of its binding to PTH was mutated and used as a negative antigen for subsequent identification of antibody characteristics. Construction of wild-type and mutant human PTH1R ECD-hFc plasmids using pFUSE as a vector ( figure 1 ), the human Fc tag is beneficial to the purification of the antibody by the Protein A adsorption column. Using the purified wild-type and mutant human PTH1R ECD-hFc protein, it is expected that the antibody that can compete with PTH for binding to the key site of PTH1R can be obtained. Blocks the interaction of PTH with PTH1R.
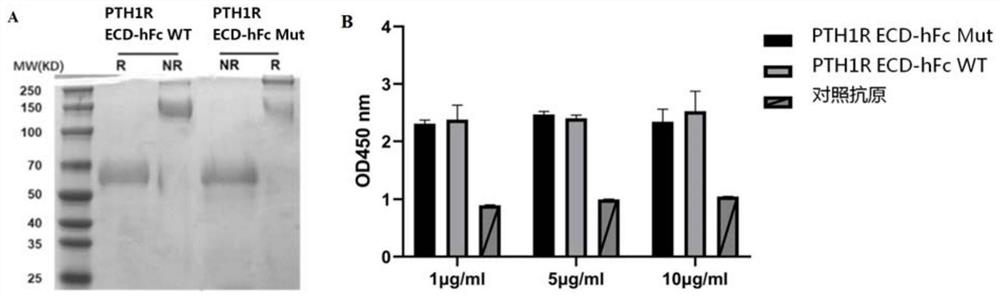
[0035] 1.2 Identification of wild-type and mutant human PTH1R ECD-hFc proteins
[0036] The purified wild-type and mutant human PTH1R ECD-hFc proteins were verified by agarose gel electrophoresis experiments to verify the molecular weight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com