A kind of tissue culture method of aseptic vaccine
A technology for tissue culture and sterile seedlings, applied in the field of plant tissue culture, can solve problems such as difficulty in reproduction, difficulty in obtaining plant seedlings, etc., and achieve the effects of overcoming difficulty in reproduction, a simple and easy tissue culture method, and a high plant survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step 1: Prepare the primary medium and proliferation medium
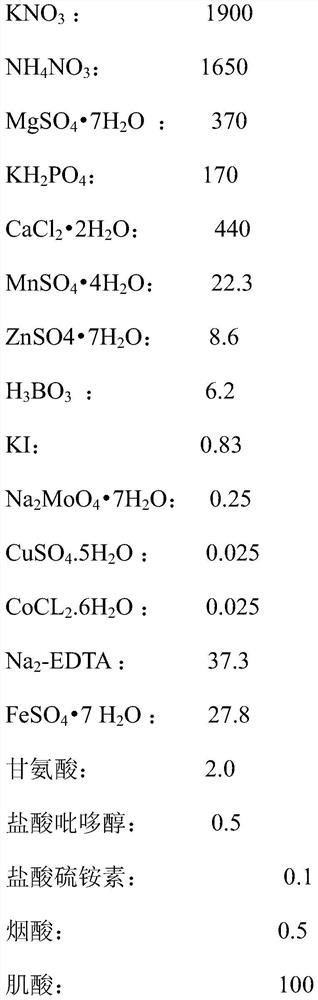
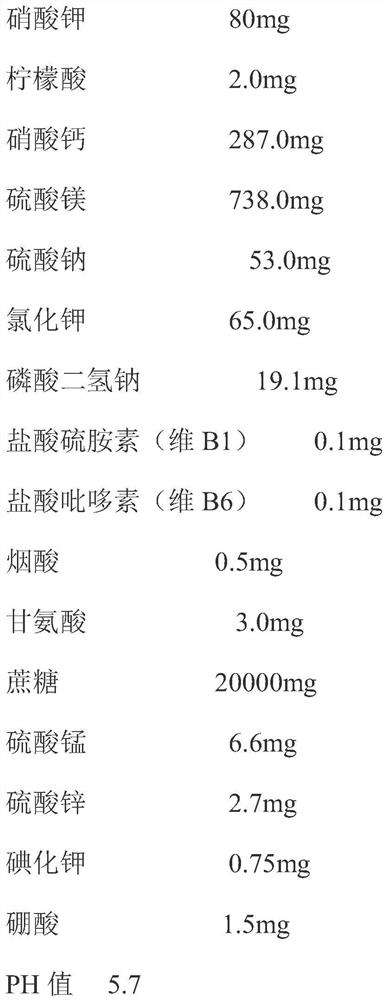
[0027] 1. The primary culture medium and proliferation medium prepared in this example are MS medium, and the formula is as follows: mg / L
[0028]
[0029] 2. Preparation of primary culture medium
[0030] Add 7g of agar, 30g of sucrose, 0.5mg of 6-aminopurine (6BA), and 0.1mg of naphthaleneacetic acid (NAA) to 1L of the above MS medium to prepare a solid medium, and sterilize it at 98kPa and 115-125°C for 20min before use; The medium was divided into 150ml tissue culture bottles, 30ml in each bottle.
[0031] 3. Preparation of proliferation medium
[0032] Add 7g of agar, 30g of sucrose, 0.5mg of kinetin (KT), and 0.1mg of naphthaleneacetic acid (NAA) to 1L of the above-mentioned MS medium to prepare a solid medium, and sterilize it at 98kPa and 115-125°C for 20min before use; Base fractions were packed into 150ml tissue culture bottles, 30ml per bottle.
[0033] Step 2: Cleaning and disinfection of e...
Embodiment 2
[0043] Step 1: Prepare the primary medium and proliferation medium
[0044] 1. The primary medium and proliferation medium prepared in this example are MS medium, and the formula is the same as that shown in Example 1.
[0045] 2. Preparation of primary culture medium
[0046] Add 7g of agar, 30g of sucrose, 1mg of 6-aminopurine (6BA) and 0.3mg of naphthaleneacetic acid (NAA) to 1L of the above MS medium to prepare a solid medium, and sterilize it at 98kPa and 115-125°C for 20min before use; Base fractions were packed into 150ml tissue culture bottles, 30ml per bottle.
[0047] 3. Preparation of proliferation medium
[0048] Add 7g of agar, 30g of sucrose, 1.25mg of kinetin (KT), and 0.3mg of naphthaleneacetic acid (NAA) to 1L of the above-mentioned MS medium to prepare a solid medium, and sterilize it at 98kPa and 115-125°C for 20min before use; Base fractions were packed into 150ml tissue culture bottles, 30ml per bottle.
[0049] Step 2: Cleaning and disinfection of exp...
Embodiment 3
[0059] Step 1: Prepare the primary medium and proliferation medium
[0060] 1. The primary medium and proliferation medium prepared in this example are MS medium, and the formula is the same as that shown in Example 1.
[0061] 2. Preparation of primary culture medium
[0062] Add 7g of agar, 30g of sucrose, 1.5mg of 6-aminopurine (6BA), and 0.5mg of naphthaleneacetic acid (NAA) to 1L of the above MS medium to prepare a solid medium, and sterilize it at 98kPa and 115-125°C for 20min before use; The medium was divided into 150ml tissue culture bottles, 30ml in each bottle.
[0063] 3. Preparation of proliferation medium
[0064] Add 7g of agar, 30g of sucrose, 2mg of kinetin (KT), and 0.5mg of naphthaleneacetic acid (NAA) to 1L of the above MS medium to prepare a solid medium, and sterilize it at 98kPa and 115-125°C for 20min before use; Divide into 150ml tissue culture bottles, 30ml per bottle.
[0065] Step 2: Cleaning and disinfection of explants
[0066] Purchase the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com