Preparation method and applications of nickel cobaltate
A technology of nickel cobaltate and nickel salt, which is applied in chemical instruments and methods, nickel compounds, inorganic chemistry, etc., can solve the problems of poor rate characteristics of structural materials, and achieve the effect of solving poor rate characteristics and significant technological progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
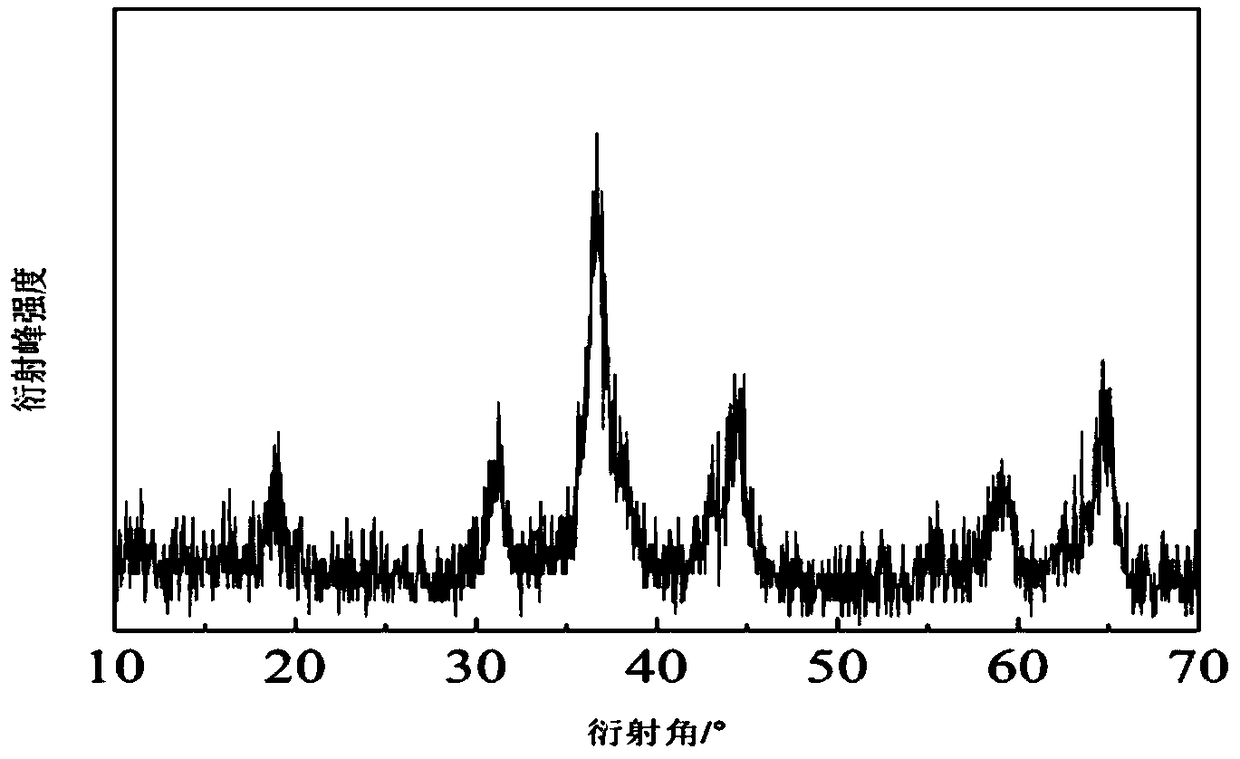
[0033] Get 0.5821g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O, analytically pure, Sinopharm Chemical Reagent Company), 1.1641g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O, analytically pure, Sinopharm Chemical Reagent Company), 0.8411g hexamethylenetetramine (C 6 h 12 N 4 , analytically pure, Sinopharm Chemical Reagent Company) was added to 60ml deionized water, wherein Ni(NO 3 ) 2 :Co(NO 3 ) 2 :C 6 h 12 N 4 : The molar ratio of deionized water is 1:2:3:266.67, and the whole adding process is carried out under magnetic stirring (81-2 type, Shanghai Sile Instrument Co., Ltd.). After stirring for 40min, the entire mixed solution was moved into a polytetrafluoroethylene-lined autoclave (100ml, Zhengxin Instrument Factory), the filling degree was 60%, and it was reacted at a certain temperature (100° C.) for 6h, then naturally cooled. get the reaction solution;
[0034]Centrifuge the above reaction solution, control the centrifugation speed to 3300r / min, and the time is 20min. The...
Embodiment 2
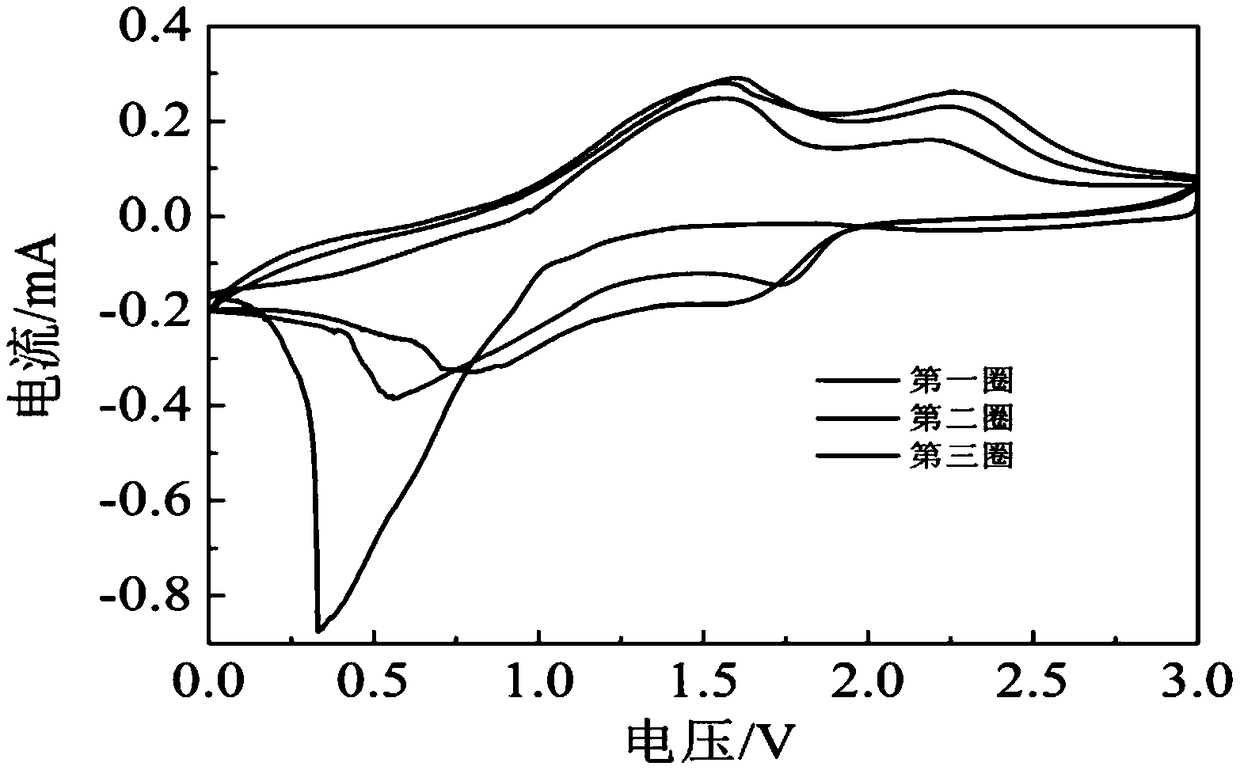
[0038] Get 0.5821g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O, analytically pure, Sinopharm Chemical Reagent Company), 1.1641g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O, analytically pure, Sinopharm Chemical Reagent Company), 0.8411g hexamethylenetetramine (C 6 h 12 N 4 , analytically pure, Sinopharm Chemical Reagent Company) was added to 75ml deionized water, wherein Ni(NO 3 ) 2 :Co(NO 3 ) 2 :C 6 h 12 N 4 : The molar ratio of deionized water is 1:2:3:333.34, and the whole adding process is carried out under magnetic stirring (81-2 type, Shanghai Sile Instrument Co., Ltd.). After stirring for 40min, the whole mixed solution was moved into a polytetrafluoroethylene-lined autoclave (100ml, Zhengxin Instrument Factory), the filling degree was 75%, and reacted at a certain temperature (100°C) for 6h, then cooled naturally, get the reaction solution;
[0039] Centrifuge the above reaction solution, control the centrifugation speed to 3300r / min, and the time is 20min. The obtained...
Embodiment 3
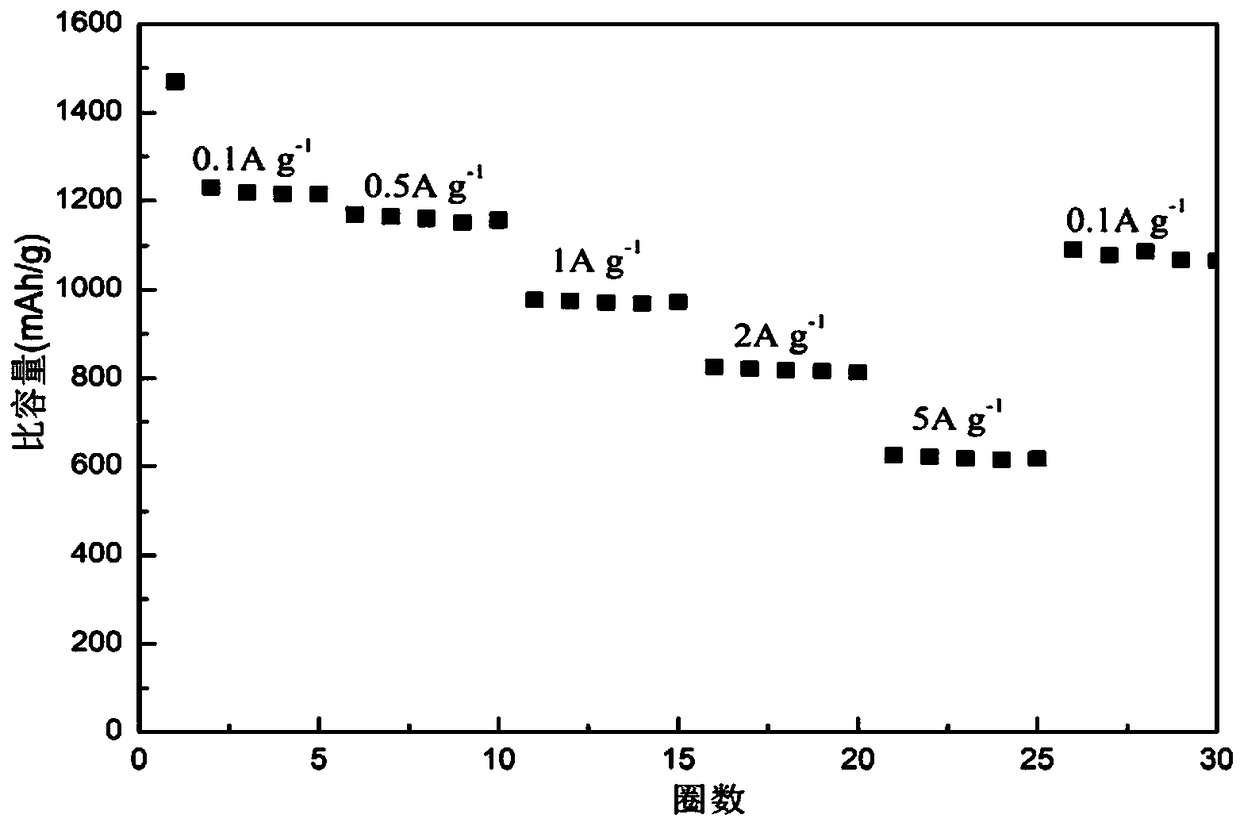
[0043] Get 0.5821g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O, analytically pure, Sinopharm Chemical Reagent Company), 1.1641g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O, analytically pure, Sinopharm Chemical Reagent Company), 0.8411g hexamethylenetetramine (C 6 h 12 N 4 , analytically pure, Sinopharm Chemical Reagent Company) was added to 70ml deionized water, wherein Ni(NO 3 ) 2 :Co(NO 3 ) 2 :C 6 h 12 N 4 : The molar ratio of deionized water is 1:2:3:311.12, and the whole adding process is carried out under magnetic stirring (81-2 type, Shanghai Sile Instrument Co., Ltd.). After stirring for 40min, the entire mixed solution was moved into a polytetrafluoroethylene-lined autoclave (100ml, Zhengxin Instrument Factory), the filling degree was 70%, and it was reacted at a certain temperature (120° C.) for 7h, then naturally cooled. get the reaction solution;
[0044] Centrifuge the above reaction solution, control the centrifugation speed to 3300r / min, and the time is 20min. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com