Preparation and Application of Herbicidal Compounds with Different Substituents of Phenol Esters
A compound and a secondary substitution technology, applied in the field of pesticides, can solve problems such as insufficient duration, high cost, and poor weeding efficacy, and achieve the effects of reducing dosage and cost, reducing residues, and mitigating potential threats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
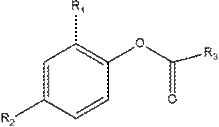
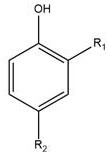
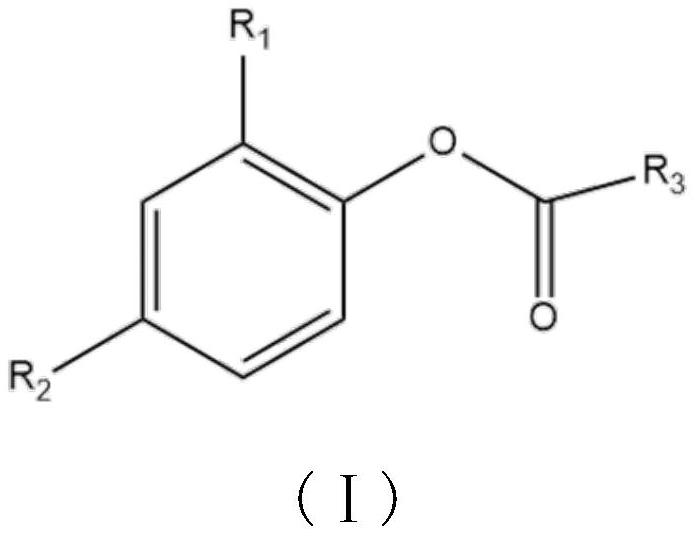
[0031] This example provides a 2,4-disubstituted phenol organic acid ester: nonanoic acid-2-nitro-4-methylphenyl ester, the preparation method is as follows:
[0032] 3.0g (19.6mmol) of 2-nitro-4-methylphenol was dissolved in 30mL of N,N-dimethylformamide, 3.8g (21.6mmol) of nonanoyl chloride was added, 2.18g of triethylamine (21.6mmol ), refluxed for 3-4h, cooled and poured into the ice water of ammonium carbonate, stirred for 1h, left standstill, precipitated solid, filtered to obtain nonanoic acid-2-nitro-4-methylphenyl ester, dried and weighed 5.38g, produced Rate: 85%.
[0033] The relevant data are as follows: 1HNMR (400MHz, DMSO-d6) δ: 7.80 (d, 1H), 7.53 (d, 1H), 7.40 (d, 1H), 2.81 (t, 2H), 2.23 (s, 3H), 1.70 (m, 2H), 1.42 (m, 2H), 1.38 (m, 2H), 1.30 (m, 6H), 0.9 (s, 3H). MS: (M+Na)+: 316.18
Embodiment 2
[0035] This example provides a 2,4-disubstituted phenol organic acid ester: 2-bromo-4-sulfonic acid phenyl dodecanoate, the preparation method is as follows:
[0036] 3.0g (13.7mmol) of 2-bromo-4-sulfonic acid phenol, dissolved in 30mL of dimethyl sulfoxide, added 3.3g (15mmol) of dodecanoyl chloride, 1.65g of triethylamine (16.5mmol), and refluxed for 3-4h , cooled and poured into ice water of ammonium carbonate, stirred for 1 h, allowed to stand still, the solid precipitated, filtered to obtain 2-bromo-4-sulfonic acid phenyl dodecanoate, weighed 3.35g after drying, yield: 61%.
[0037] The relevant data are as follows: 1HNMR (400MHz, DMSO-d6) δ: 9.10 (br, 1H), 8.81 (d, 1H) 8.33 (dd, 1H), 7.90 (dd, 1H), 2.78 (t, 2H), 2.13 ( m, 2H), 1.70 (m, 2H), 1.38 (m, 2H), 1.32 (m, 2H), 1.28 (m, 10H), 0.9 (s, 3H). MS: (M+Na)+: 424.66
Embodiment 3
[0039]This example provides a 2,4-disubstituted phenol organic acid ester: 2,4-hexadienoic acid 2-bromo-4-nitrophenyl ester, the preparation method is as follows:
[0040] 5.0g (23mmol) of 2-nitro-4-methylphenol was dissolved in 30mL of dimethyl sulfoxide, and 3.0g (23mmol) of 2,4-hexadienoyl chloride, 2.55g of triethylamine (25.3mmol) were added , refluxed for 3-4h, cooled and poured into ice water with ammonium carbonate, stirred for 1h, stood still, precipitated solid, filtered to obtain 2-bromo-4-nitrophenyl 2,4-dihexenoic acid, weighed 5.56 g, Yield: 78%.
[0041] The relevant data are as follows: 1HNMR (400MHz, DMSO-d6) δ: 8.68 (d, 1H), 7.83 (dd, 1H), 7.41 (d, 1H), 7.52 (d, 1H), 6.33 (m, 1H), 6.10 (d, 1H), 5.44 (m, 1H), 1.7 (d, 3H), 1.30 (m, 6H), 0.9 (s, 3H). MS: (M+Na)+: 334.06
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com