Application of avobenzone in the preparation of antitumor drugs
An anti-tumor drug, avobenzone technology, applied in the field of medicine, can solve the problems of not verifying the therapeutic effect of avobenzone on skin cancer, researching and verifying the significance of skin cancer treatment, and not having a cell biology perspective, etc. Clinical drug resistance, improving application value, and clear effect of countermeasures for side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
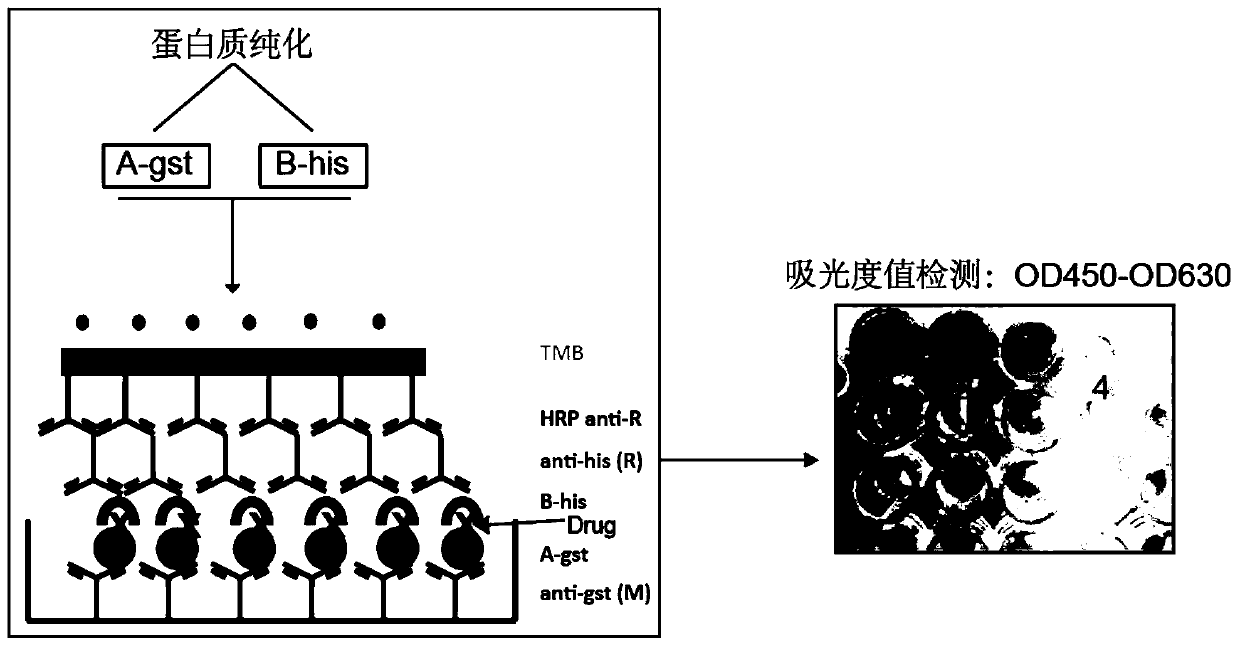
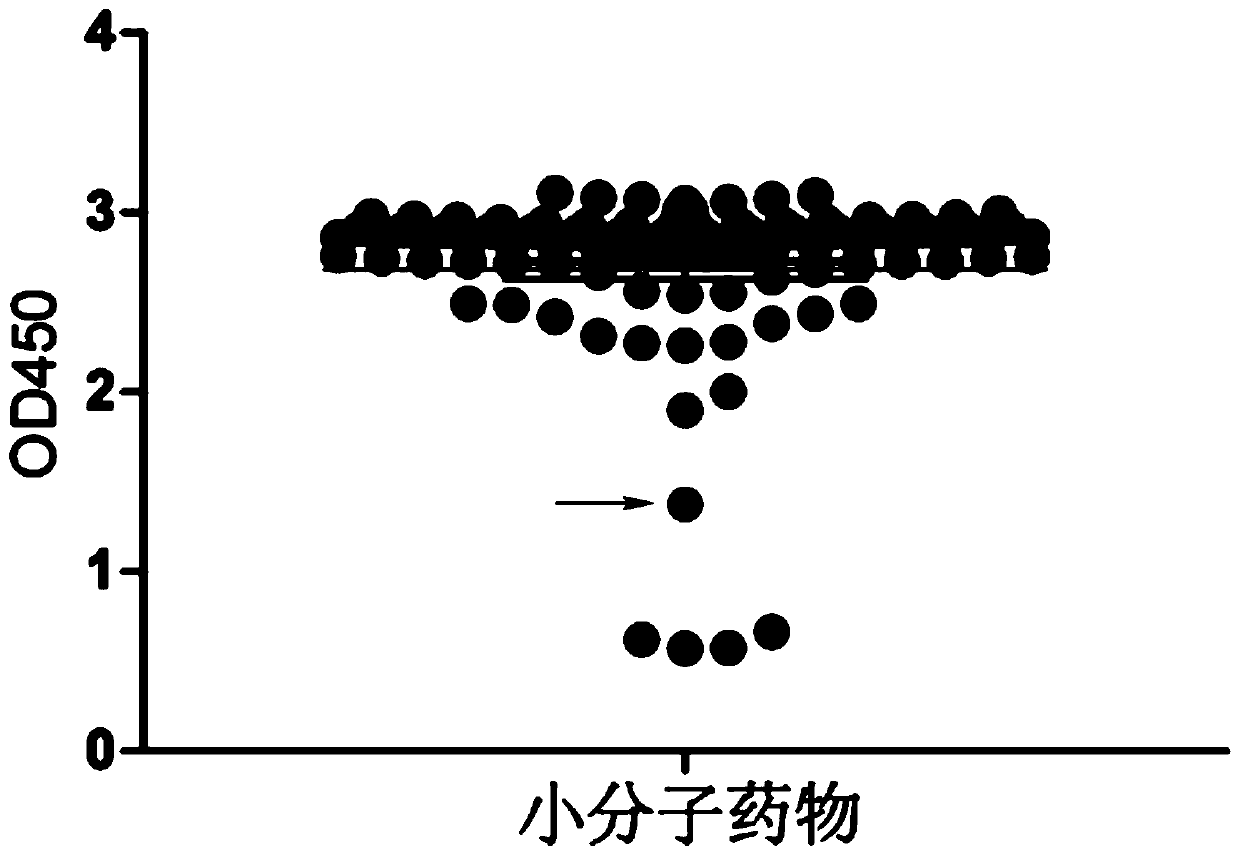
[0026] Example 1 Research on the anti-tumor mechanism of Avobenzone by superELISA technology
[0027] 1. Preparation of fusion protein PHB1-his and C-Raf1-gst
[0028] (1) Expression and purification of PHB1-his fusion protein
[0029] 1. Construction of the recombinant expression vector pET-his: According to the human PHB1 protein gene sequence of NCBI database (NCBI accession number: NM_001281496.1), primers encoding PHB1 gene were designed and synthesized by Primer Premier 5.0, and double restriction sites were added at both ends Point (upstream end restriction site is BamH1, downstream end restriction site is EcoR1, both purchased from TAKARA), PCR amplification of the target gene band.
[0030] 2. Use 1% agarose gel electrophoresis to detect the PCR product, and use the DNA recovery kit (Tiangen Biochemical Technology Co., Ltd., Item No. DP214) to recover and purify the target fragment. Use endonuclease to double digest the target gene fragment and the empty vector pET (Biyuntia...
Embodiment 2
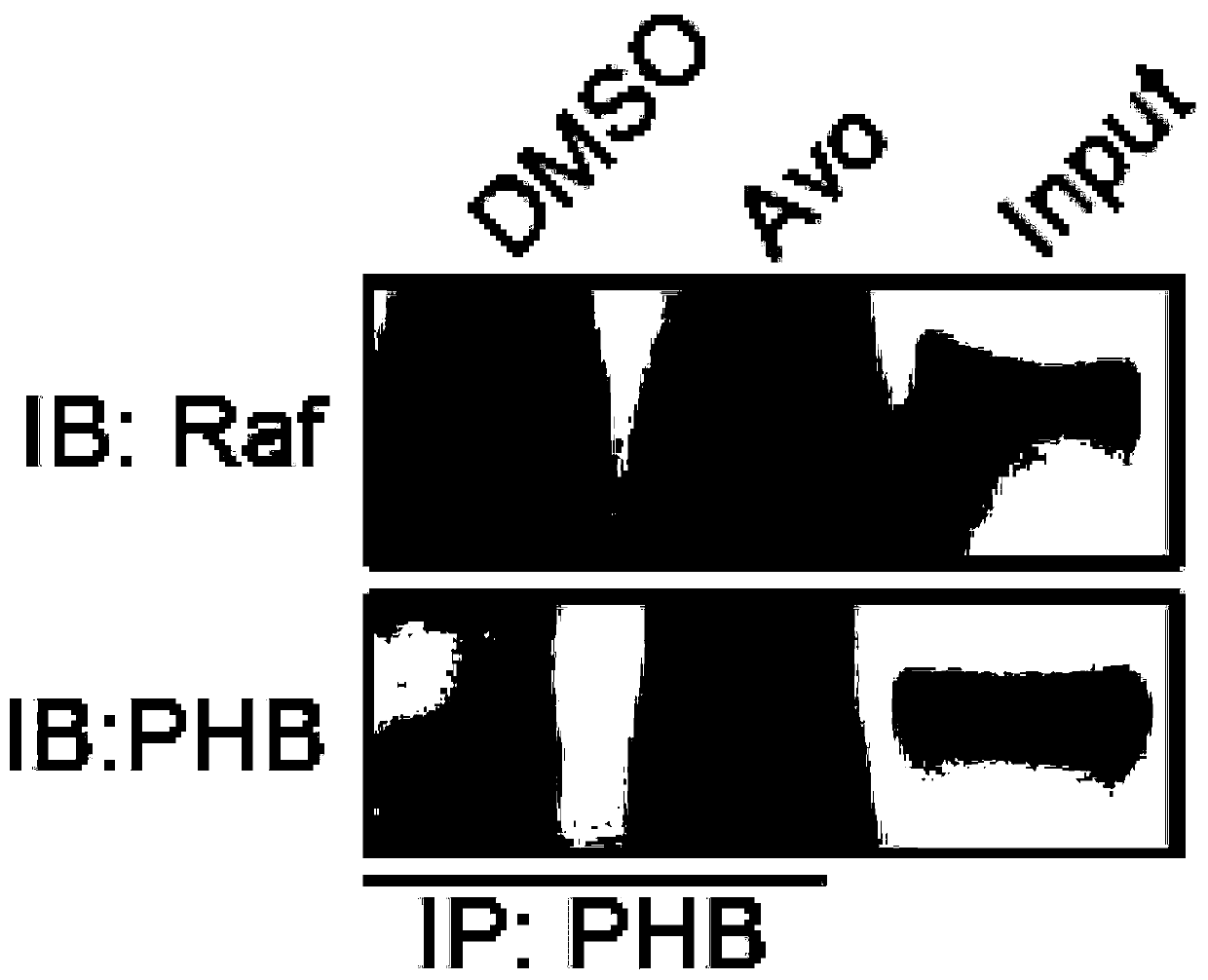
[0070] Example 2 Co-immunoprecipitation experiment
[0071] Co-immunoprecipitation experiment (Co-IP) is an important means to test the interaction of proteins in vivo. In order to further study and verify the anti-cancer mechanism and effect of avobenzone, the co-immunoprecipitation experiment (Co-IP) was carried out in this example. The role of Zong is further studied. The specific operation steps of Co-IP are as follows:
[0072] (1) Two groups of 1 million colon cancer HCT116 cells in each group were plated in a cell culture dish in advance, and DMSO and avobenzone were added respectively. DMSO is added in the ratio of 1μL:1000μL DMEM culture solution, and Avobenzone is diluted with DMEM culture solution, and the final concentration is 10μM;
[0073] (2) Incubate the treated cells at 37°C for 24 hours, then wash the cells twice with PBS, add 1 mL of 0.25% trypsin to each culture dish to make the cells fall off the culture dish wall; then terminate with 5 mL of complete culture ...
Embodiment 3
[0082] Example 3 Study of MEK activation level
[0083] Protein MEK is a downstream molecule of PHB-c-Raf1 signaling pathway. By detecting the activation level of MEK, the phosphorylation state (pMEK), it can be confirmed whether the interaction between PHB and c-Raf1 is disrupted. The decrease of pMEK level also means that the metastatic ability of colon cancer cells is decreased.
[0084] Detect the activation level of MEK by western blot, the specific steps are as follows:
[0085] 1. Pave colon cancer cell HCT116 or colon cancer cell RKO (purchased from ATCC, article number CRL-2577) in a six-well plate with 500,000 cells per well. Add 2μL each of DMSO (control group) and avobenzone (experimental group) to the culture wells, and add 2000μL DMEM complete culture solution to each well. The final concentration of avobenzone is 10μM. Mix the culture medium and incubate the treated cells at 37°C for 48 hours.
[0086] 2. Wash the cells twice with PBS, add 0.5mL 0.25% trypsin to each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com