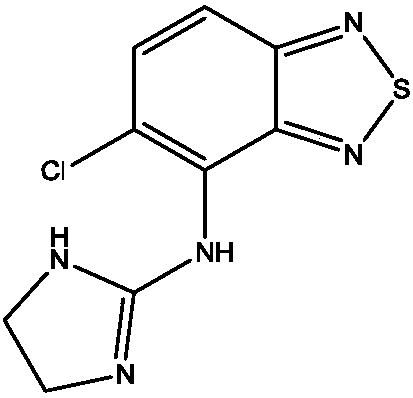
Tizanidine hydrochloride sustained-release preparation, preparation process and uses thereof
A technology for tizanidine hydrochloride and sustained-release preparations, applied in the field of tizanidine hydrochloride sustained-release tablets, can solve the problems of high blood drug concentration and obvious side effects, and achieve low blood drug concentration, small side effects, and bioavailability high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
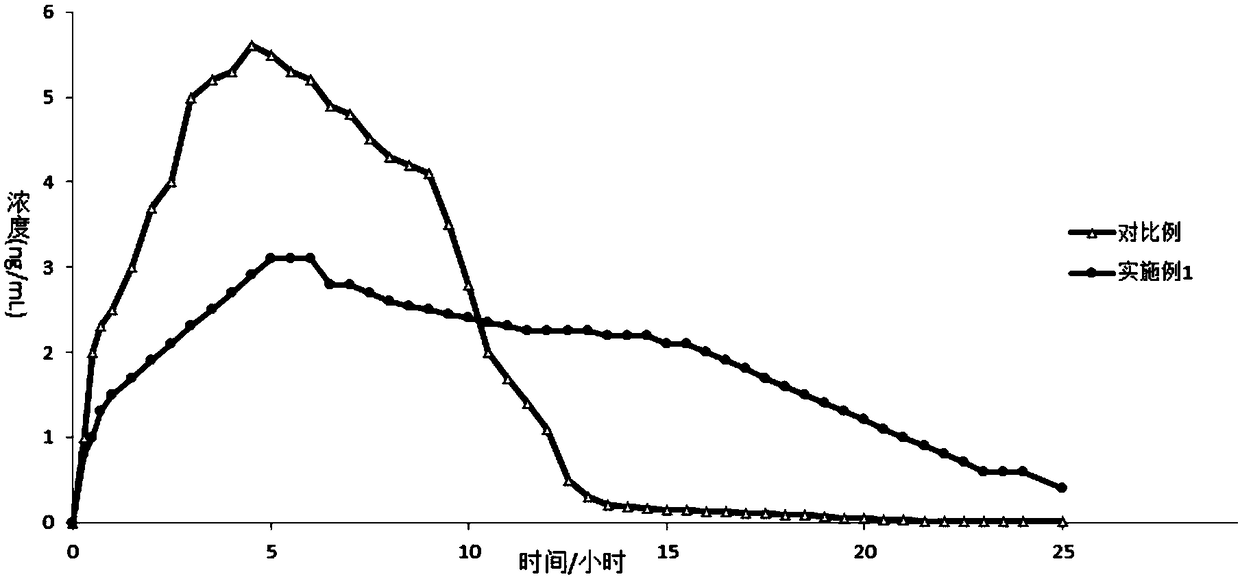
Image
Examples
Embodiment 1
[0028] Tizanidine Hydrochloride
[0029] Grind and mix tizanidine hydrochloride, HPMC K100M, microcrystalline cellulose and pregelatinized starch, add PVP K30 solution prepared with 90% ethanol to make soft material, granulate, dry, granulate, add hard Magnesium fatty acid and micropowder silica gel are mixed evenly, and pressed into tablets to obtain the product.
Embodiment 2
[0031]
[0032]
[0033] The preparation method is the same as in Example 1.
Embodiment 3
[0035] Tizanidine Hydrochloride
[0036] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com