Composite tumor antibody vaccine using bacterial nano-magnetosome as carrier, and preparation method thereof
A nano-magnetic and bacterial technology, applied in the direction of antibodies, anti-tumor drugs, chemical instruments and methods, etc., to achieve a strong anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
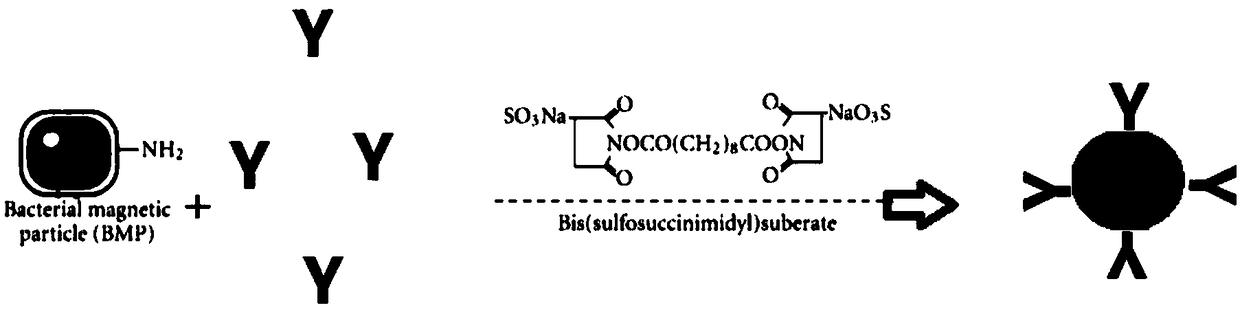
[0036] Preparation of compound tumor antibody vaccine (BMP-anti-4-1BB):
[0037] (1) For pure bacterial nano-magnetosomes (preparation methods: Sun JB, Zhao F, Tang T, Jiang W, TianJS, Li Y Li JL. High-yield growth and magnetosome formation by Magnetospirillum gryphiswaldense MSR-1 in an oxygen-controlled fermentor supplied solely with air.2008; 79(3):389-397) is sterilized by γ-ray irradiation, and the irradiation dose is 15kGy; according to the method of Nakamura, N. et al. Absorbance value (Nakamura, N., Mutsunaga, T. Highly sensitive detection of allergen using bacterial magnetic particles: Biosensors. Anal Chem Acta, 1993, 281, 585-589) and quantification according to 1OD660 = 172 μg of bacterial nano magnetosomes.
[0038] (2) Anti-4-1BB monoclonal antibody was prepared according to Zhou H et al. (Zhou H, Zhang GB, Dong QM, Yu GH, Xu Y, Zhang XG. Preparation of anti-human 4-1BB monoclonal antibody and characterization of its Biological activities, Xi Bao Yu Fen Zi Mian ...
Embodiment 2
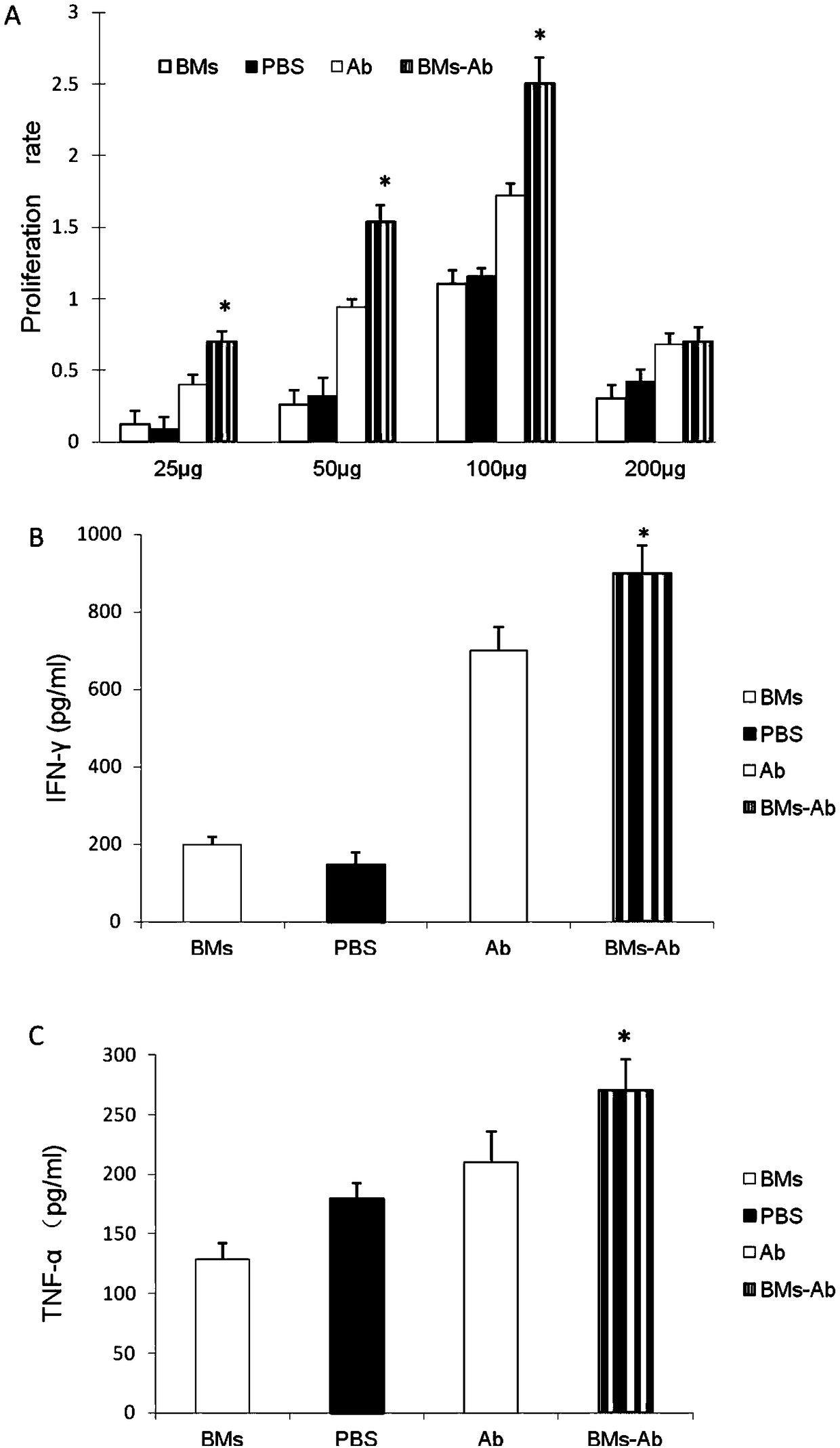
[0041] Selection of Optimal Dose of Compound Tumor Antibody Vaccine Stimulating T Cells
[0042] (1) Preparation of compound tumor antibody vaccine: (see the corresponding part of Example 1 for specific operation method)
[0043] (2) Fresh spleen cells of mice were obtained from C57BL / 6 mice. After removing red blood cells with Tris solution (Beijing Zhongshan Jinqiao Biological Co., Ltd.), the splenocyte solution was passed through a nylon mesh to make a single splenocyte solution. 25 μg, 50 μg, 100 μg and 200 μg of compound tumor antibody vaccine were used as stimulators, and the concentration was 2×10 5Splenocytes / ml were used as reactive T cells. After the reactive T cells and stimulators were added to each well of the 96-well incubation plate, a 600mT magnet was placed under each well for 10 minutes. The 96-well plate to which the mixture was added was then placed in a chamber containing 5% CO 2 37°C incubator. After 72 hours, detect with CCK8 reagent, read the OD va...
Embodiment 3
[0045] Effect of compound tumor antibody vaccine on stimulating CD8+ T cells
[0046] (1) Isolation and purification of CD8+ T cells from mice
[0047] CD8+ T cells were isolated from the spleen of C57BL / 6 mice using CD8+ T cell MACS isolation and purification kit (Miltenyi, Biotec). The biotin-labeled monoclonal antibody mixture was combined with non-CD8+ T cells, and then the non-CD8+ T cells were removed by indirect labeling magnetic beads method, and the purity of the remaining CD8+ T cells was detected by CD8-PE antibody in flow cytometry.
[0048] (2) Detection of cytokines
[0049] Add the CD8+T cells isolated in the above steps into a 96-well plate, add 1×105CD8+T cells to each well, and then add 100 μg of compound tumor antibody vaccine, and then use a 600mT magnet to act on each well for 10 minutes, and incubate in carbon dioxide. After 48 hours of co-cultivation in the box, the supernatant was collected, and the concentrations of IFN-γ and TNF-α were determined wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com