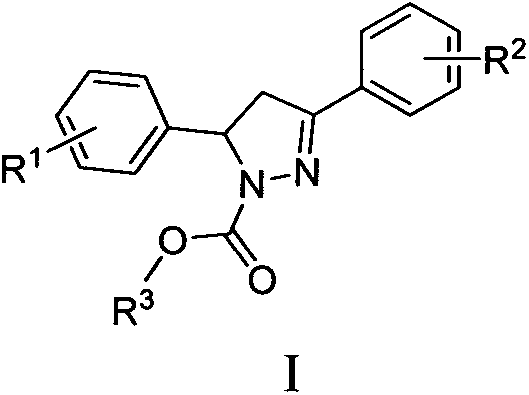
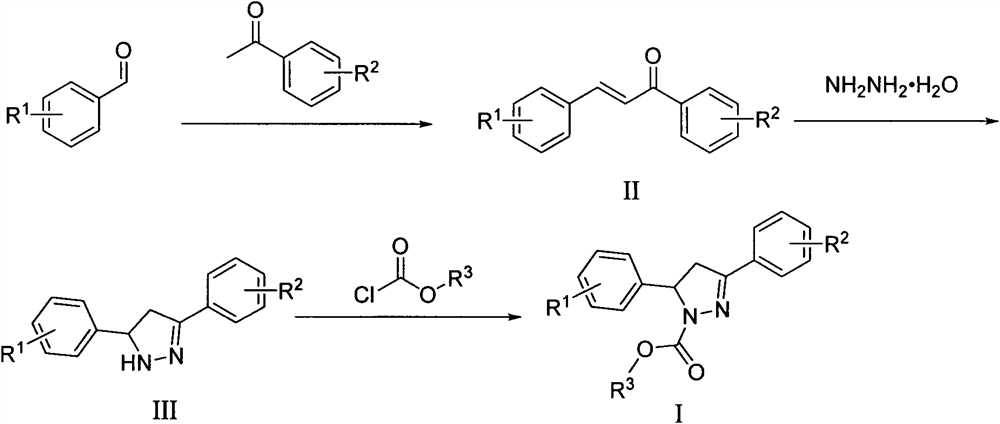
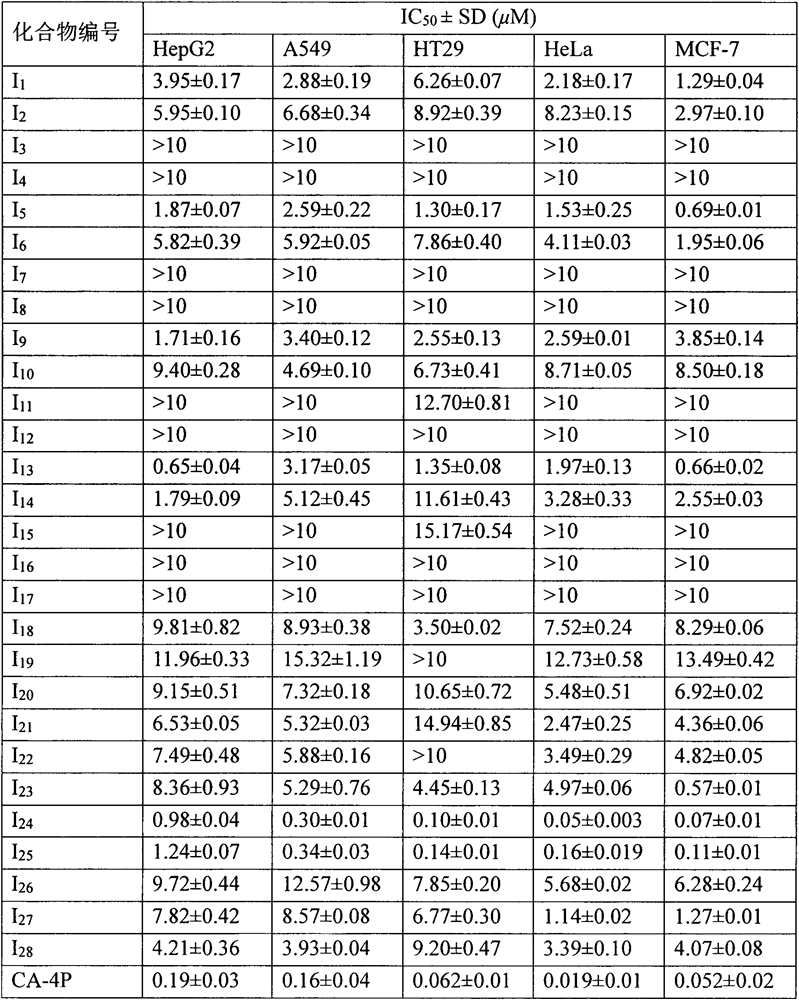
A kind of microtubule polymerization inhibitor containing pyrazoline structure and its application
A technology of pyrazolines and structural formulas, applied in the preparation of anti-tumor drugs, in the field of new microtubule polymerization inhibitors, can solve the problems of large toxic and side effects, poor curative effect of solid tumors, multi-drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: 3-phenyl-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carboxylic acid methyl ester (I 1 ) preparation
[0059] The synthesis steps are: ① 3,4,5-trimethoxybenzaldehyde (392mg, 2.0mmol) and acetophenone (240mg, 2.0mmol) were dissolved in 20mL of absolute ethanol, KOH (6mmol, 336mg) was added, stirred at room temperature, and reacted After 24 hours, a solid substance was precipitated, suction filtered, washed, and recrystallized to obtain the intermediate product II 1 ; ② intermediate product II 1 (596 mg, 2.0 mmol) and 1 mL of 80% hydrazine hydrate solution were dissolved in 5 mL of isopropanol solution, and stirred and refluxed for 3 hours under nitrogen protection. After the temperature of the reaction solution dropped to room temperature, it was transferred to cold hydrazine, stirred at -20°C until a large amount of solids were precipitated, filtered under reduced pressure in a vacuum, and the solids were quickly washed three times with saturated s...
Embodiment 2
[0060] Example 2: 3-(3-methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carboxylic acid methyl ester (I 5 ) preparation
[0061] For the synthesis method, refer to Example 1. Obtain white powder, productive rate 61.2%, melting point 164-166 ℃, nuclear magnetic spectrum is characterized as 1 H-NMR (DMSO-d 6 , 600MHz) δ7.39-7.30(m, 2H), 7.26(s, 1H), 7.04-7.02(m, 1H), 6.49(s, 2H), 5.39(dd, J=11.9, 5.4Hz, 1H) , 3.87-3.78 (m, 4H), 3.73 (s, 6H), 3.67 (s, 3H), 3.63 (s, 3H), 3.18 (dd, J=17.9, 5.5Hz, 1H).
Embodiment 3
[0062] Example three: 3-(3-hydroxyphenyl)-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carboxylic acid methyl ester (I 13 ) preparation
[0063] For the synthesis method, refer to Example 1. Obtain white powder, productive rate 77.1%, melting point 137-139 ℃, nuclear magnetic spectrum is characterized as 1 H-NMR (DMSO-d 6 , 600MHz) δ9.63(s, 1H), 7.25-7.20(m, 2H), 7.12(d, J=7.6Hz, 1H), 6.85(d, J=7.6Hz, 1H), 6.48(s, 2H ), 5.37(dd, J=11.8, 5.4Hz, 1H), 3.79(dd, J=17.8, 11.9Hz, 1H), 3.73(s, 6H), 3.66(s, 3H), 3.63(s, 3H) , 3.10 (dd, J=17.8, 5.3Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com