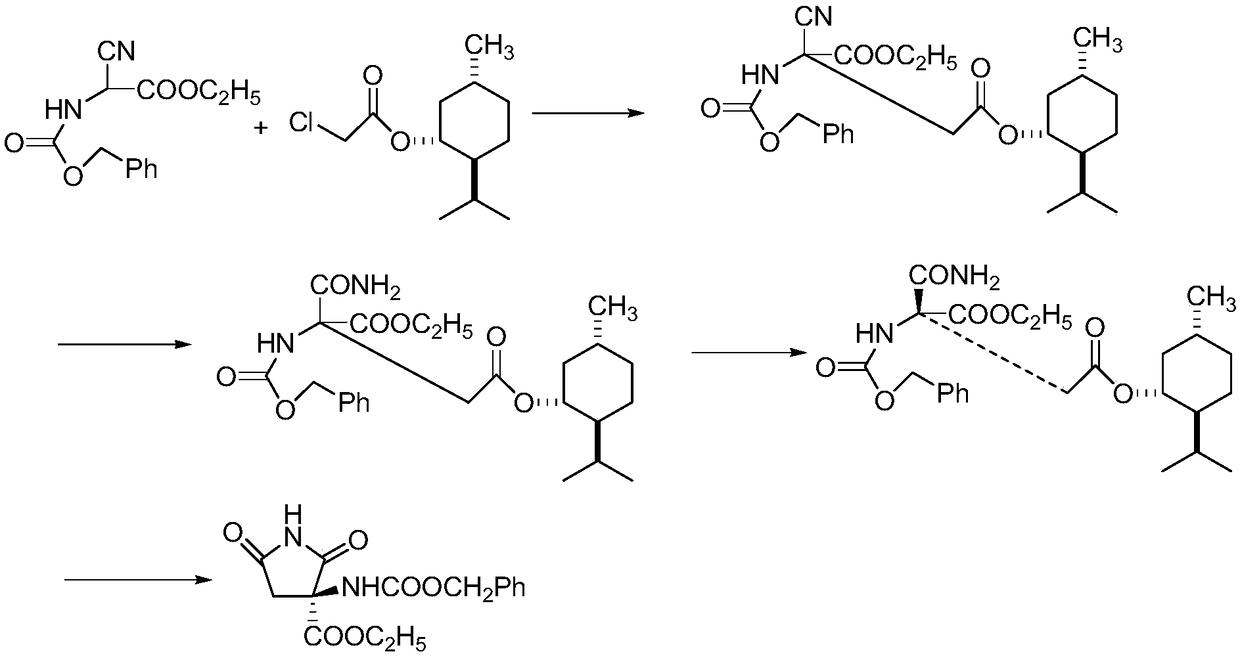
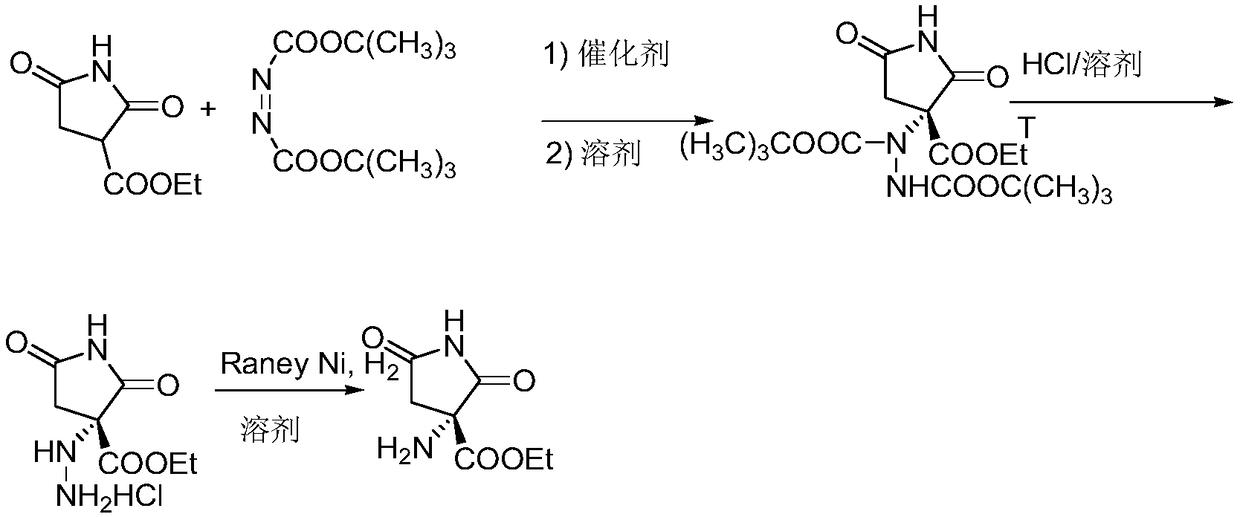
Synthesis method of ethyl R-3-amino-2,5-dioxopyrrole-3-carboxylate
A technology of ethyl carboxylate and dioxypyrrole, which is applied in the field of synthesis of R-3-amino-2,5 dioxypyrrole-3-ethyl carboxylate, can solve problems such as difficulty in preparation, and achieve improved chiral induction The effect of realizing asymmetric Michael addition reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthetic method of R-3-amino-2,5-dioxypyrrole-3-carboxylic acid ethyl ester, realizes through the following steps:
[0045] 1) Preparation of R-3-di-tert-butoxyhydrazide-1-benzyl-2,5-dioxypyrrolidine-3-carboxylic acid ethyl ester
[0046] Add 2.61g (0.01mol) of ethyl 1-benzyl-2,5-dioxypyrrolidine-3-carboxylate, square amide (ⅢA) (0.45g, 0.001mol), azodicarboxylate 2.48 g (0.0108 mol) of di-tert-butyl acid and 15 mL of dry dichloromethane were reacted at -20°C. The reaction was followed by TLC for 10 hours. After the reaction was complete, the solvent was removed by concentration under reduced pressure, and the residue was directly used in the next reaction.
[0047] Optical purity analysis of the residue: HPLC Chiracel AD-H, λ=254nm
[0048] Mobile phase n-hexane:isopropanol=9:1
[0049] Column temperature: 30°C
[0050] Retention time: 8.5(S) and 11.6min(R)
[0051]ee% of product = 80.8%
[0052] 2) Preparation of R-3-hydrazino-1-benzyl-2,5-dioxypyrrolidine-3...
Embodiment 2
[0061] The synthetic method of R-3-amino-2,5-dioxypyrrole-3-carboxylic acid ethyl ester, realizes through the following steps:
[0062] 1) Preparation of R-3-di-tert-butoxyhydrazide-1-benzyl-2,5-dioxypyrrolidine-3-carboxylic acid ethyl ester
[0063] Add 2.61g (0.01mol) of ethyl 1-benzyl-2,5-dioxypyrrolidine-3-carboxylate, square amide (ⅢB) (0.48g, 0.001mol), azodicarboxylate 2.48 g (0.0108 mol) of di-tert-butyl acid and 15 mL of dry dichloromethane were reacted at -20°C. The reaction was followed by TLC for 10 hours. After the reaction was complete, the solvent was removed by concentration under reduced pressure, and the residue was directly used in the next reaction. The optical purity of the residue was analyzed ee% = 87.8%.
[0064] 2) Preparation of R-3-hydrazino-1-benzyl-2,5-dioxypyrrolidine-3-carboxylic acid ethyl ester hydrochloride
[0065] Add ethyl acetate (10mL) to the residue to dissolve, slowly add trifluoroacetic acid (4.56g) in ethyl acetate solution (8mL) dr...
Embodiment 3
[0069] The synthetic method of R-3-amino-2,5-dioxypyrrole-3-carboxylic acid ethyl ester, realizes through the following steps:
[0070] 1) Preparation of R-3-di-tert-butoxyhydrazide-1-benzyl-2,5-dioxypyrrolidine-3-carboxylic acid ethyl ester
[0071] Add 2.61 g (0.01 mol) of ethyl 1-benzyl-2,5-dioxypyrrolidine-3-carboxylate, square amide (ⅢC) (0.49 g, 0.001 mol), azodicarboxy 2.48 g (0.0108 mol) of di-tert-butyl acid and 15 mL of dry dichloromethane were reacted at -20°C. The reaction was followed by TLC for 10 hours. After the reaction was complete, the solvent was removed by concentration under reduced pressure, and the residue was directly used in the next reaction. Optical purity analysis of the residue: ee% = 97.5%
[0072] 2) Preparation of R-3-hydrazino-1-benzyl-2,5-dioxypyrrolidine-3-carboxylic acid ethyl ester hydrochloride
[0073] Add dichloromethane (10mL) to the residue to dissolve, slowly add 5M HCL in dichloromethane solution (8mL) dropwise, and react at room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com