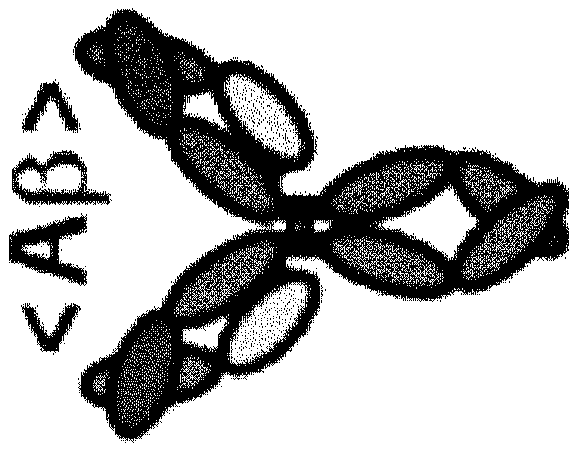
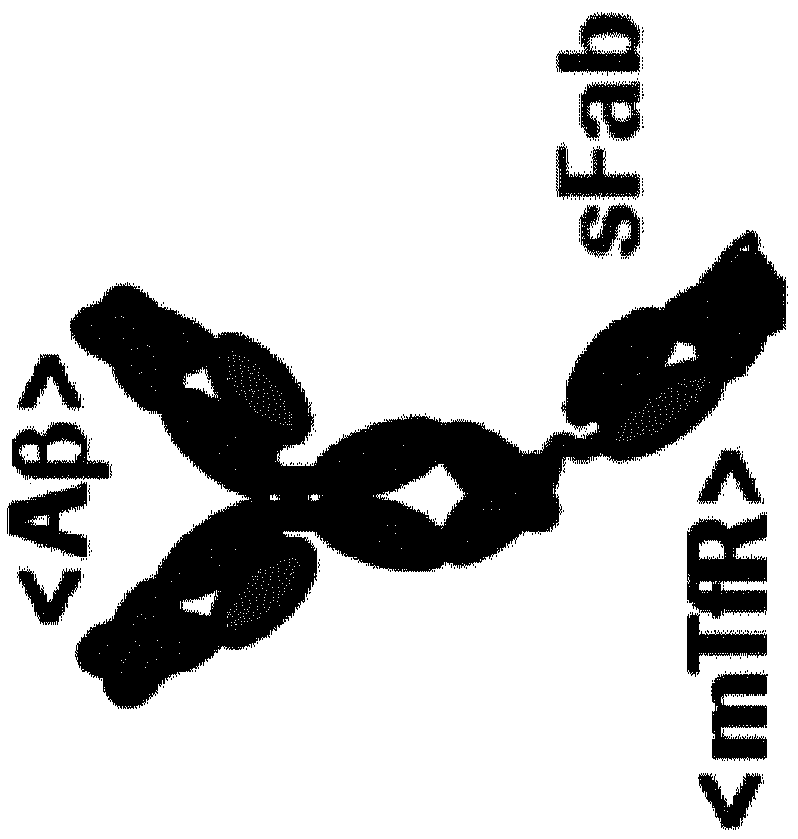
Blood brain barrier shuttle
A blood-brain barrier and entity technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, for targeting specific cell fusion, etc., can solve problems such as limiting pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Embodiment 1: Preparation of expression plasmid
[0158] Description of Basic / Standard Mammalian Expression Plasmids
[0159] The desired protein was expressed by transient transfection of human embryonic kidney cells (HEK 293). In order to express the desired gene / protein (e.g. antibody-Fab multimeric protein), a transcription unit comprising the following functional elements is used:
[0160]Immediate early enhancer and promoter from human cytomegalovirus (P-CMV), including intron A,
[0161] ●Human heavy chain immunoglobulin 5'-untranslated region (5'UTR),
[0162] Mouse immunoglobulin heavy chain signal sequence (SS),
[0163] the gene / protein to be expressed (e.g. full-length antibody heavy chain), and
[0164] • Bovine growth hormone polyadenylation sequence (BGH pA).
[0165] In addition to the expression unit / cassette including the desired gene to be expressed, the basic / standard mammalian expression plasmid contains:
[0166] an origin of replication fr...
Embodiment 2
[0198] Example 2: Purification of single and double Mab31-Fab constructs
[0199] Antibody chains were prepared by transient transfection of HEK293 cells (derived from the human embryonic kidney cell line 293) cultured in F17 medium (Invitrogen Corp.). For transfection, "293-Fectin" transfection reagent (Invitrogen) was used. Expression of antibody chains from 2 (tetravalent Mab31-scFab(8D3)) or 3 (trivalent Mab31-scFab(8D3)) different plasmids encoding the tetravalent Mab31-scFab(8D3) heavy chain and the Mab31 counterpart Light chain, or overhang and hole trivalent Mab31-scFab(8D3) heavy chain and Mab31 corresponding light chain. After transfection, the 2 or 3 plasmids were used in equimolar plasmid ratios. Transfection was performed as described in the manufacturer's instructions. Seven days after transfection, cell culture supernatants containing antibody fusion proteins were harvested. Supernatants were stored frozen until purification.
[0200] Proteins were purified...
Embodiment 3
[0201] Example 3: ELISA binding data for single and double Fab constructs
[0202] Binding of mAb31-8D3 constructs to mouse transferrin receptor (mTfR) was assessed by indirect ELISA. For this purpose, recombinant mTfR (extracellular domain; Sino Biological) was coated onto Maxisorb microtiter plates (Nunc) at 1 μg / mL in PBS overnight at 4°C. After blocking in 1% Crotein-C / PBS (blocking buffer; Roche) for 1 h at room temperature and washing 4 times with 0.1% Tween-20 / PBS (washing buffer), the mAb31-8D3 construct was blocked in blocking buffer. Concentrations between 0.01-150 nM in ® were added to the wells and incubated for 1 h at room temperature. After 4 washing steps, the construct was detected by adding anti-human-IgG-HRP (Jackson Immunoresearch) at a 1:10,000 dilution in blocking buffer (1RT), followed by 6 washes and warming in TMB (Sigma) education. After stopping the color development with 1N HCl, the absorbance was read at 450 nm.
[0203] image 3 showed that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com