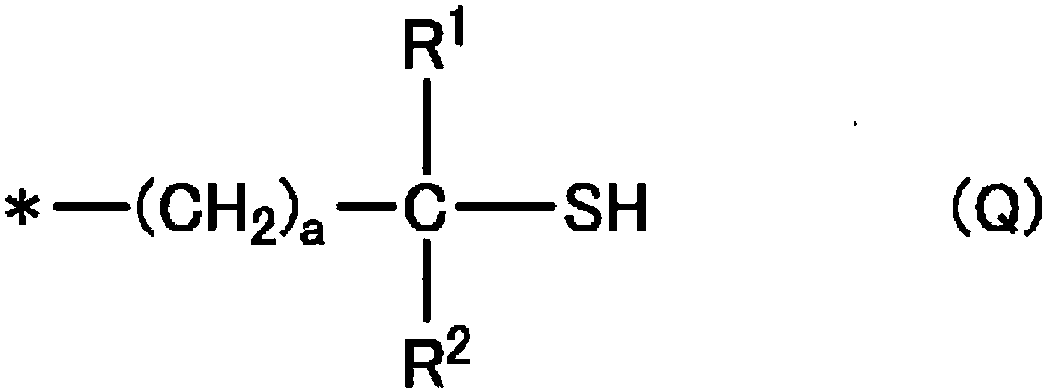
Composition for carbon fiber-reinforced resin, carbon fiber-reinforced resin composition, cured article
A technology for reinforcing resin and carbon fiber, which is applied in the field of carbon fiber reinforced resin composition and its cured product, and carbon fiber reinforced resin composition, which can solve the problems of insufficient adhesion and insufficient interlaminar shear strength of the cured product. achieve good adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7
[0282] Mixture of each (A) radical reactive resin in (A1-1) (A1-2) (A4-1) produced by the above method, and (B) styrene as a radically polymerizable unsaturated monomer , the (B) radically polymerizable unsaturated monomer shown in Table 1 was added as needed. Thereby, the mixture (mixture of (A) component and (B) component) containing (A) radical reactive resin and (B) radically polymerizable unsaturated monomer in the ratio shown in Table 1 was obtained.
[0283] [Table 1]
[0284]
[0285] In the column of styrene in Table 1, each (A) radical reactive resin and (B) radical polymerizable unsaturated monomer of the above-mentioned (A1-1) (A1-2) (A4-1) The content of styrene contained in the mixture beforehand and the content of styrene added as (B) radically polymerizable unsaturated monomer as needed are summed up and described.
[0286] In addition, in the column of (A) radical reactive resin in Table 1, it is described that in the mixture calculated from the addition ...
Embodiment 8~9、 comparative example 2
[0299] In the same manner as in Example 1, a mixture containing (A) a radically reactive resin and (B) a radically polymerizable unsaturated monomer at the ratio shown in Table 2 was obtained.
[0300] [Table 2]
[0301]
[0302] In the column of styrene in Table 2, each (A) radical reactive resin and (B) radical polymerizable unsaturated monomer of the above-mentioned (A1-1) (A1-2) (A4-1) The content of styrene previously contained in the mixture of , and the content of styrene added as (B) radically polymerizable unsaturated monomer as needed are summed up and described.
[0303] In addition, in the (A) radical reactive resin column in Table 2, only (A) in the mixture calculated from the addition amount of the raw material used for the manufacture of the mixture of (A) component and (B) component is described. Content of free radical reactive resin. (A) The content of the radical reactive resin was calculated assuming that 100% of the raw materials used in the manufactu...
Embodiment 10~13
[0320] In the same manner as in Example 1, a mixture containing (A) a radically reactive resin and (B) a radically polymerizable unsaturated monomer at the ratio shown in Table 3 was obtained.
[0321] [table 3]
[0322]
[0323] In the styrene column in Table 3, each (A) radical reactive resin of (A1-1) (A1-2) (A2-1) (A4-1) mentioned above was polymerized with (B) radical The content of styrene previously contained in the mixture of non-saturated unsaturated monomers and the content of styrene added as (B) radically polymerizable unsaturated monomers if necessary are summed up and described.
[0324] In addition, in the (A) radical reactive resin column in Table 3, only (A) in the mixture calculated from the addition amount of the raw material used for the manufacture of the mixture of (A) component and (B) component is described. Content of free radical reactive resin. (A) The content of the radical reactive resin was calculated assuming that 100% of the raw materials u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com