Sinomenine derivative, preparation method and application thereof and pharmaceutical composition
A derivative, sinomenine technology, applied in the preparation of salt, inflammatory diseases and aplastic anemia in the field of application, to achieve good pharmacodynamic characteristics, significant anti-inflammatory activity, the effect of preventing and treating aplastic anemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
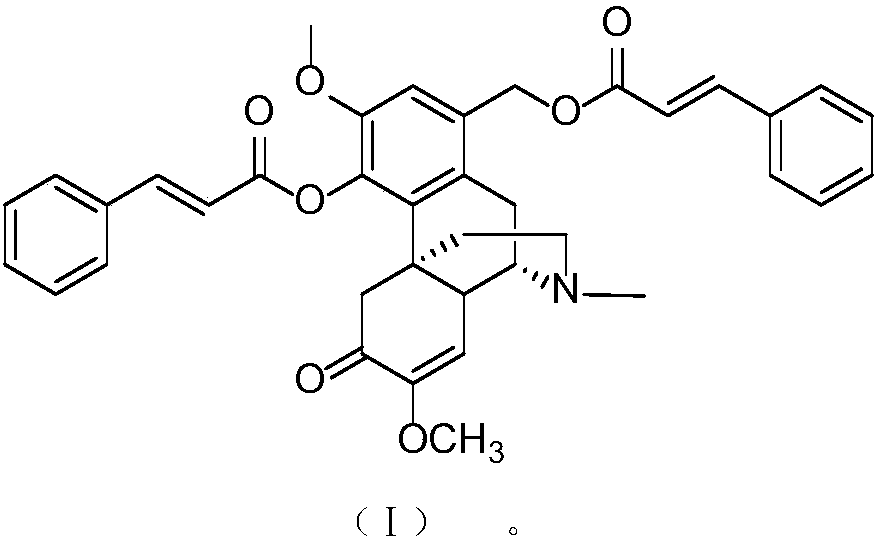
[0033] Embodiment 1: the preparation of sino-wcj-43
[0034] Molecular formula: C 38 h 37 NO 7
[0035] Nomenclature: 1-cinnamoyloxymethylene-4-cinnamoyloxy-7,8-didehydro-3,7-dimethoxy-17-methyl-morphinan-6-one, 1-cinnamoyloxymethylene-4-cinnamoyloxy-7, 8 Didehydro-3,7-dimethoxy-17-methyl-morphinan-6-one.
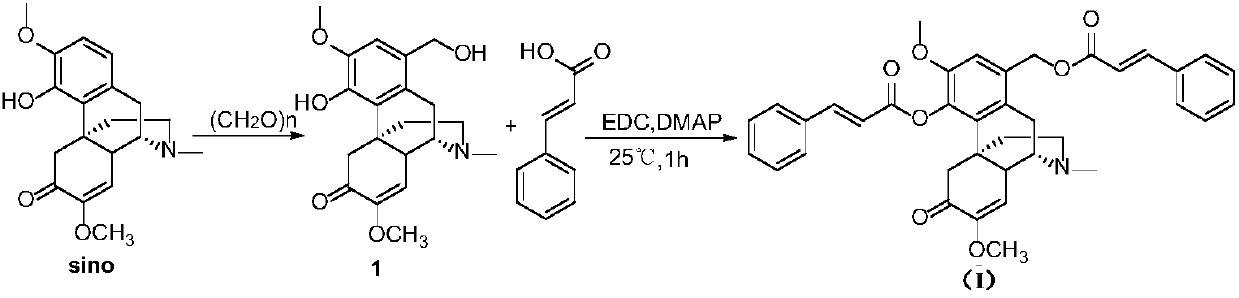
[0036] Compound synthesis route:
[0037]
[0038] Step 1: Weigh 10.0 g of sinomenine hydrochloride, dissolve it in 200 ml of water, add 20.0 g of paraformaldehyde (2-3 times the mass of sinomenine hydrochloride) under stirring, heat and reflux for 4h-12h, and monitor the reaction process by TLC. After the reaction, use CHCl 3 Extraction (100mL × 3 or the number of extractions can be increased), combined organic phase, anhydrous Na 2 SO 4 After drying, filtering, and evaporating off the solvent, a white solid was obtained, and 1-hydroxymethylene sinomenine was obtained.
[0039] Step 2, take about 50 mg of 1-hydroxymethylene sinomenine, dissolve it in dichlorome...
experiment example 1
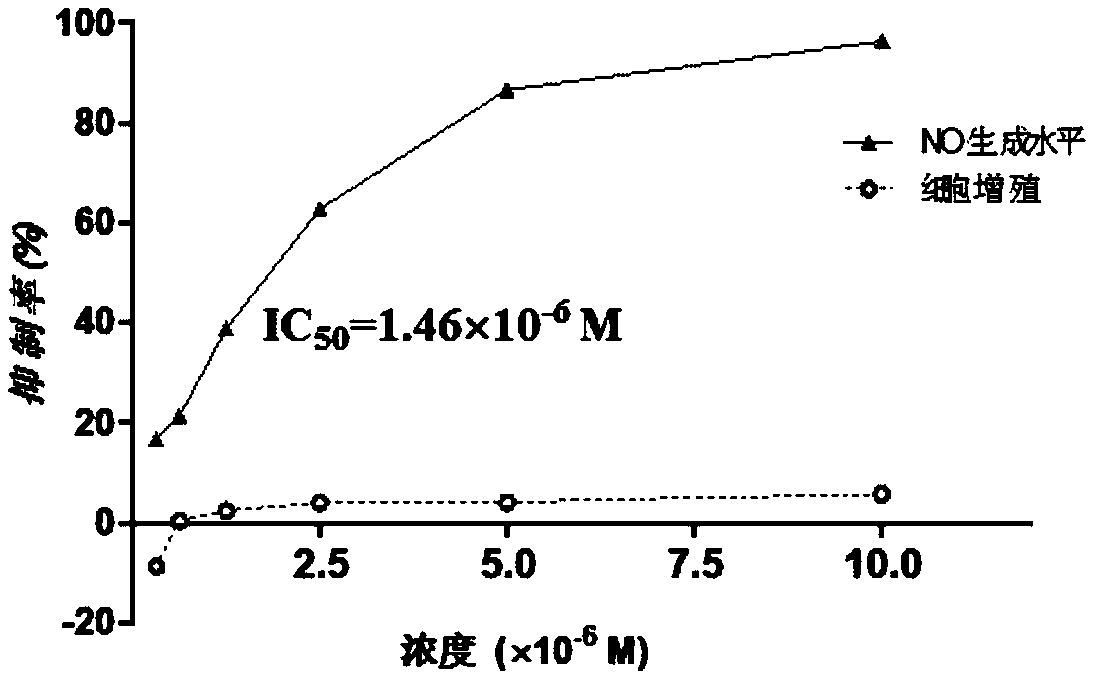
[0042] Experimental example 1: Inhibitory effect of sino-wcj-43 on LPS-induced production of inflammatory proton NO in primary mouse peritoneal macrophages
[0043] Macrophages perform the body's non-specific immune function. Under the induction of bacterial lipopolysaccharide LPS, they can produce inflammatory factors such as NO, participate in and mediate the inflammatory response, and have high levels in the initial stage of various inflammatory immune processes and in the process of pathological development. Level. By detecting the amount of NO produced by primary cultured mouse peritoneal macrophages, it can be used as a preliminary observation of the anti-inflammatory activity of the compound in vitro.
[0044] experimental method:
[0045] Primary mouse peritoneal macrophages were inoculated in 96-well plates, and compounds of different concentrations were added for pre-protection for 1 h; then, LPS was added to make the final concentration 1 μg / ml, and the cells were ...
experiment example 2
[0050] Experimental example 2: Effect of sino-wcj-43 on otitis induced by croton oil in mice
[0051] experimental method:
[0052] Take 18-20g male Kunming mice, divide them into groups randomly, and apply 0.02ml of croton oil on both sides of the left ear of each group of animals respectively; 30 minutes later, each group of animals is subcutaneously injected with 50mg / kg and 100mg / kg body weight of the test compound respectively , the model control group was given an equal volume of vehicle; 4 hours after the administration, the mice were killed by neck dislocation, and both ears were cut off along the baseline of the auricles, and the ear pieces at the same positions of the left and right ears were removed with a 6 mm diameter punch, and weighed with an analytical balance. Calculate ear swelling degree (ear swelling degree=left ear piece weight-right ear piece weight) and ear swelling inhibition rate [ear swelling inhibition rate (%)=(model group average ear swelling degre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com