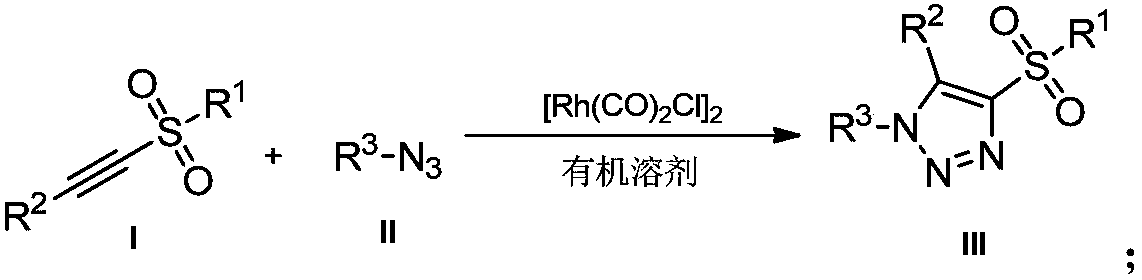
Novel method for preparing 4-sulfonyl-1,4,5-tri-substituted 1,2,3-triazole
A sulfonyl, tri-substituted technology, applied in the field of organic synthesis, can solve problems such as no public reports, and achieve the effects of high reaction efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
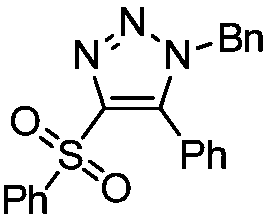
[0020] Example 1: Preparation of 4-benzenesulfonyl-(1-benzyl)-5-phenyl-1H-1,2,3-triazole
[0021] Under air, 1-benzenesulfonylphenylacetylene (0.2mmol, 48.4mg) was dissolved in 1,2-dichloroethane (2mL), then benzyl azide (0.3mmol, 40.2mg) and [Rh (CO) 2 Cl] 2 (0.005mmol, 1.9mg), the reaction mixture was stirred at 40°C, and reacted for 12h. After the reaction, 61mg of a colorless oily product was obtained by column chromatography, with a yield of 81%.
[0022]
[0023] 1 H NMR (400MHz, CDCl 3 ,TMS):δ7.79(d,J=8.0Hz,2H),7.55-7.50(m,2H),7.44-7.39(dd,J=12.0,8.0Hz,4H),7.24-7.19(m,3H ), 7.11(d, J=8.0Hz, 2H), 6.93(d, J=8.0Hz, 2H), 5.32(s, 2H). 13 C NMR (100MHz, CDCl 3 ): δ145.9, 140.8, 139.3, 134.0, 133.8, 130.9, 130.2, 129.2, 129.0, 128.9, 128.8, 128.1, 128.0, 124.4, 52.8.
Embodiment 2
[0024] Example 2: Preparation of 4-benzenesulfonyl-(1-p-methylbenzyl)-5-phenyl-1H-1,2,3-triazole
[0025] Under air, 1-benzenesulfonylphenylacetylene (0.2mmol, 48.4mg) was dissolved in 1,2-dichloroethane (2mL), then p-methylbenzyl azide (0.3mmol, 44.1mg) was added and [Rh(CO) 2 Cl] 2 (0.005mmol, 1.9mg), the reaction mixture was stirred at 40°C, and reacted for 12h. After the reaction, 59mg of white solid product was obtained by column chromatography, with a yield of 76%.
[0026]
[0027] Mp=147-149°C. 1 H NMR (400MHz, CDCl 3 , TMS): δ7.83(d, J=8.0Hz, 2H), 7.57(t, J=8.0Hz, 2H), 7.50-7.43(m, 4H), 7.17(d, J=8.0Hz, 2H) ,7.05(d,J=8.0Hz,2H),6.87(d,J=8.0Hz,2H),5.31(s,2H),2.31(s,3H). 13 C NMR (100MHz, CDCl 3 ): δ145.7, 140.7, 139.1, 138.6, 133.6, 130.8, 130.7, 130.1, 129.5, 129.0, 128.7, 128.0, 127.8, 124.3, 52.4, 21.1. HRMS (ESI-TOF) m / z calcd for C 22 h 19 N 3 o 2 S(M+Na) + 412.1090,found 412.1094.
Embodiment 3
[0028] Example 3: Preparation of 4-benzenesulfonyl-(1-p-chlorobenzyl)-5-phenyl-1H-1,2,3-triazole
[0029] Under air, dissolve 1-benzenesulfonylphenylacetylene (0.2mmol, 48.4mg) in 1,2-dichloroethane (2mL), then add p-chlorobenzyl azide (0.3mmol, 50.1mg) and [Rh(CO) 2 Cl] 2 (0.005mmol, 1.9mg), the reaction mixture was stirred at 40°C, and reacted for 12h. After the reaction, 57mg of a yellow solid product was obtained by column chromatography, with a yield of 70%.
[0030]
[0031] Mp=132-134°C. 1 H NMR (400MHz, CDCl 3 , TMS): δ7.83(d, J=8.0Hz, 2H), 7.58(t, J=8.0Hz, 2H), 7.50-7.45(m, 4H), 7.22(d, J=8.0Hz, 2H) ,7.15(d,J=8.0Hz,2H),6.91(d,J=8.0Hz,2H),5.32(s,2H). 13 C NMR (100MHz, CDCl 3 ): δ145.9, 140.6, 139.1, 134.8, 133.7, 132.2, 130.9, 130.0, 129.3, 129.1, 128.8, 128.0, 124.1, 51.9. HRMS (ESI-TOF) m / z calcdfor C 21 h 16 ClN 3 o 2 S(M+Na) + 432.0544,found 432.0546.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com