Method for removing iron in zinc sulfate solution
A technology of zinc sulfate solution and organic complexing agent, applied in the direction of zinc sulfate, etc., can solve the problems of increasing iron ion residue, high iron removal cost, and reduced iron removal efficiency, so as to achieve improved removal rate, high iron removal efficiency, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
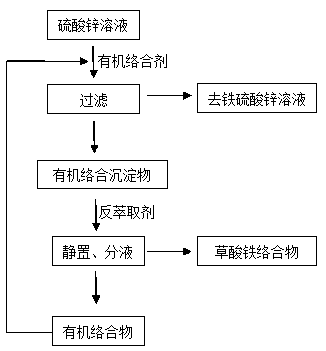
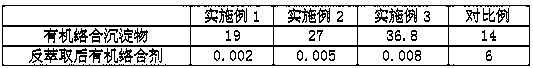
[0022] A method for removing iron in a zinc sulfate solution, the steps are: take 100mL of zinc sulfate solution, at room temperature, add a mass concentration of 30% organic Complexing agent 2mL, stirred, reacted at 30°C for 3min, filtered to obtain organic complex precipitate and iron-free zinc sulfate solution; then add 60g / L of Oxalic acid solution, back-extract the iron in the organic complex precipitate, shake for 12 minutes under the condition of shaking speed of 130rpm, let stand for 8 minutes after the shaking is completed, and separate the liquid, that is, the organic complexing agent and the iron oxalate complex, the organic complex The mixture can be recycled and used for complexing and precipitating iron in the zinc sulfate solution.
[0023] Preparation of organic complexing agent with a mass concentration of 30%: Take 30g of sodium thiocarbamate or tetrasodium iminodisuccinate in a 100mL beaker, add 70mL of water, and slightly heat to dissolve it in water.
Embodiment 2
[0025] A method for removing iron in a zinc sulfate solution, the steps are: take 150mL of zinc sulfate solution, at room temperature, add a mass concentration of 20% organic Complexing agent 3.75mL, stirred, reacted at 35°C for 3min, filtered to obtain organic complex precipitate and iron-free zinc sulfate solution; then add 55g / L oxalic acid solution, back extract the iron in the organic complex precipitate, shake for 14min under the condition of shaking speed of 120rpm, let stand for 6min after the shaking is completed, and separate the liquid to obtain the organic complexing agent and iron oxalate complex, organic The complexing agent can be recycled for complexing and precipitating iron in the zinc sulfate solution.
[0026] Preparation of organic complexing agent with a mass concentration of 20%: Take 20g of sodium formate or tetrasodium iminodisuccinate in a 100mL beaker, add 70mL of water, and slightly heat to dissolve it in water.
Embodiment 3
[0028] A method for removing iron in a zinc sulfate solution, the steps are: take 200mL of zinc sulfate solution, at normal temperature, add a mass concentration of 25% organic Complexing agent 3mL, stirred, reacted at 40°C for 4min, filtered to obtain organic complex precipitate and iron-free zinc sulfate solution; then add 50g / L of Oxalic acid solution, extract the iron in the organic complex precipitate, shake for 10 minutes under the condition of shaking speed of 120rpm, let it stand for 5 minutes after the shaking is completed, and separate the liquid, that is, the organic complexing agent and the iron oxalate complex, the organic complex The mixture can be recycled and used for complexing and precipitating iron in the zinc sulfate solution.
[0029] Preparation of organic complexing agent with a mass concentration of 25%: Take 25g of sodium formex or tetrasodium iminodisuccinate in a 100mL beaker, add 70mL of water, and slightly heat to dissolve it in water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com