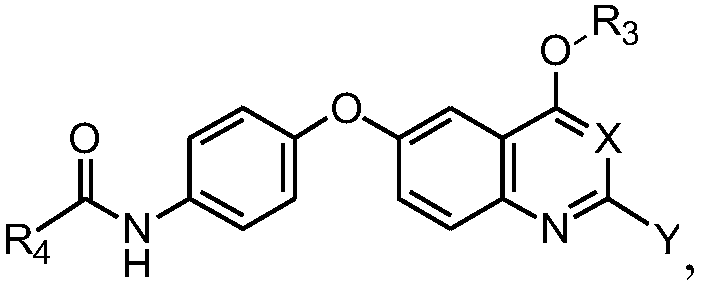
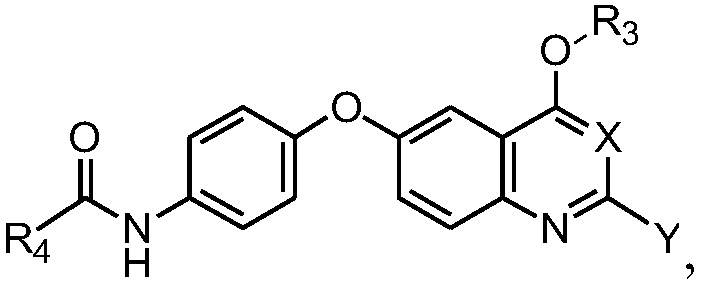
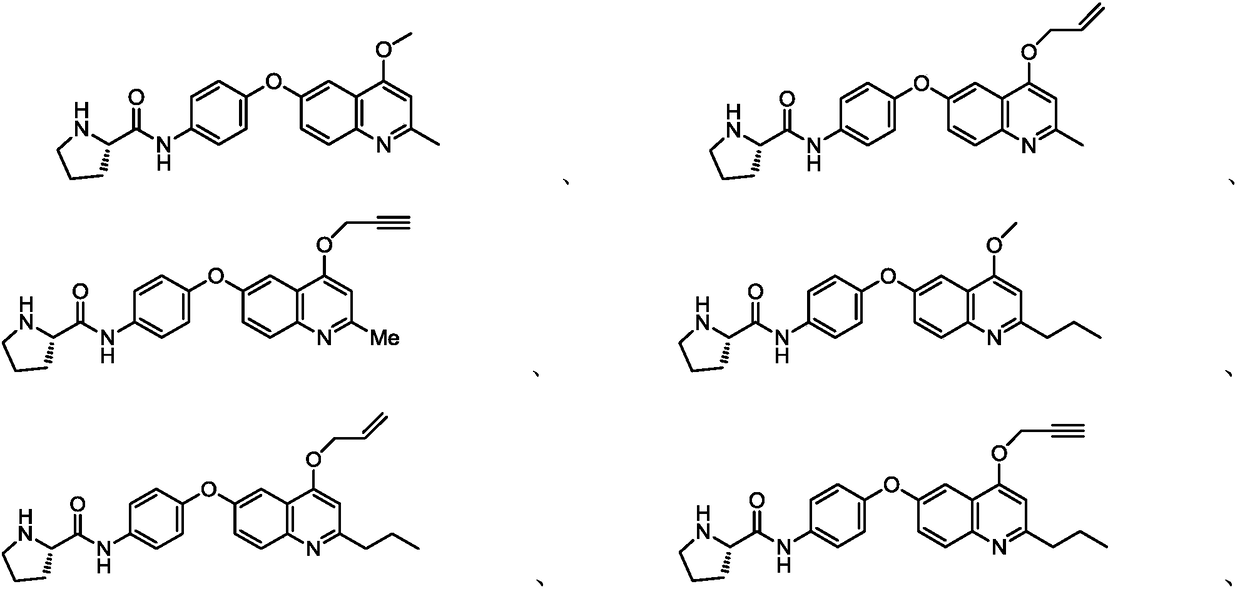
Quinoline or quinazoline compound as well as preparation method and application thereof
A technology of quinazolines and compounds, which is applied in the field of quinolines or quinazolines and their preparation, can solve the problems of lack of anti-tumor drugs, etc., and achieve simple and easy preparation methods, readily available raw materials, and less stringent preparation conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation method (method A) of the quinoline compound of one embodiment, comprises the following steps:
[0048] S110, react compound A1 with Boc anhydride to obtain compound A2.
[0049] In one of the examples, compound A1 and Boc anhydride were dissolved in methanol, and triethylamine was added under stirring, and the reaction was stirred at room temperature for 12 hours to 18 hours, and 2mol / L hydrochloric acid solution was added to obtain pure Compound A2.
[0050] Specifically, compound A1 is 4-aminophenol Its structural formula is, the chemical formula of Boc anhydride is (Boc) 2 O, the structural formula of compound A2 is
[0051] Specifically, the molar ratio of compound A1 to Boc anhydride is 1:1-1.2, preferably 1:1.2.
[0052] Specifically, the molar ratio of compound A1 to triethylamine is 1:1.5-2.3, preferably 1:2.2.
[0053] Specifically, the specific method of separation and purification is: extraction with ethyl acetate, washing with saturat...
Embodiment 1~56
[0165] Examples 1-28 used the preparation method of quinoline compounds (method A) to prepare the above-mentioned quinoline compounds. The specific parameters of the preparation are shown in Table 1, and the specific steps are as follows:
[0166] Step 1. Dissolve 20.0 mmol of 4-aminophenol and 24.0 mmol of Boc anhydride in 150 mL of methanol, add 44.0 mmol of triethylamine under stirring, and react with stirring at room temperature for 18 hours. After the reaction was completed, add 20mL of 2mol / L hydrochloric acid solution, and extract with 3×50mL ethyl acetate, combine the organic phases, wash with 2×50mL saturated brine, dry over anhydrous sodium sulfate, and concentrate in vacuo to obtain the crude product that passed through the silica gel column layer Analysis and purification (eluent: n-hexane / ethyl acetate, volume ratio 4:1) gave pure compound A2.
[0167] Step 2, 12.0 mmol of compound A2 and 10.0 mmol of 4-fluoronitrobenzene were dissolved in 50 mL of acetonitrile, ...
Embodiment 29~56
[0176] Embodiments 29-56 adopt the preparation method of quinazoline compounds (method B) to prepare the above-mentioned quinazoline compounds. The preparation parameters are shown in Table 2, and the specific steps are as follows:
[0177] Step 1, prepare compound A3 according to steps 1 and 2 in the preparation method A adopted in Examples 1 to 31, then add chloral hydrate (11.0 mmol) and 40 mL of water to the flask; then add sodium sulfate ( 80.0mmol), compound A3 (10.0mmol), hydrochloric acid solution (6mL water and 1mL concentrated hydrochloric acid configuration), and finally add the solution prepared by dissolving hydroxylamine hydrochloride (33.0mmol) in 10mL water. The reaction mixture was stirred and reacted at 110°C for 2h, heated to 130°C and stirred for 1h, cooled to room temperature, filtered, dried, and then gradually (completely added in about 20min) added to 50°C concentrated sulfuric acid (20mL) and reacted at 65°C for 2 hours. Hour. Cool to room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com