Combination treatment of ocular inflammatory disorders and diseases
An inflammatory disease, ocular technology, applied in the field of combined treatment of ocular inflammatory diseases and diseases, can solve the problems of complex immunomodulators and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
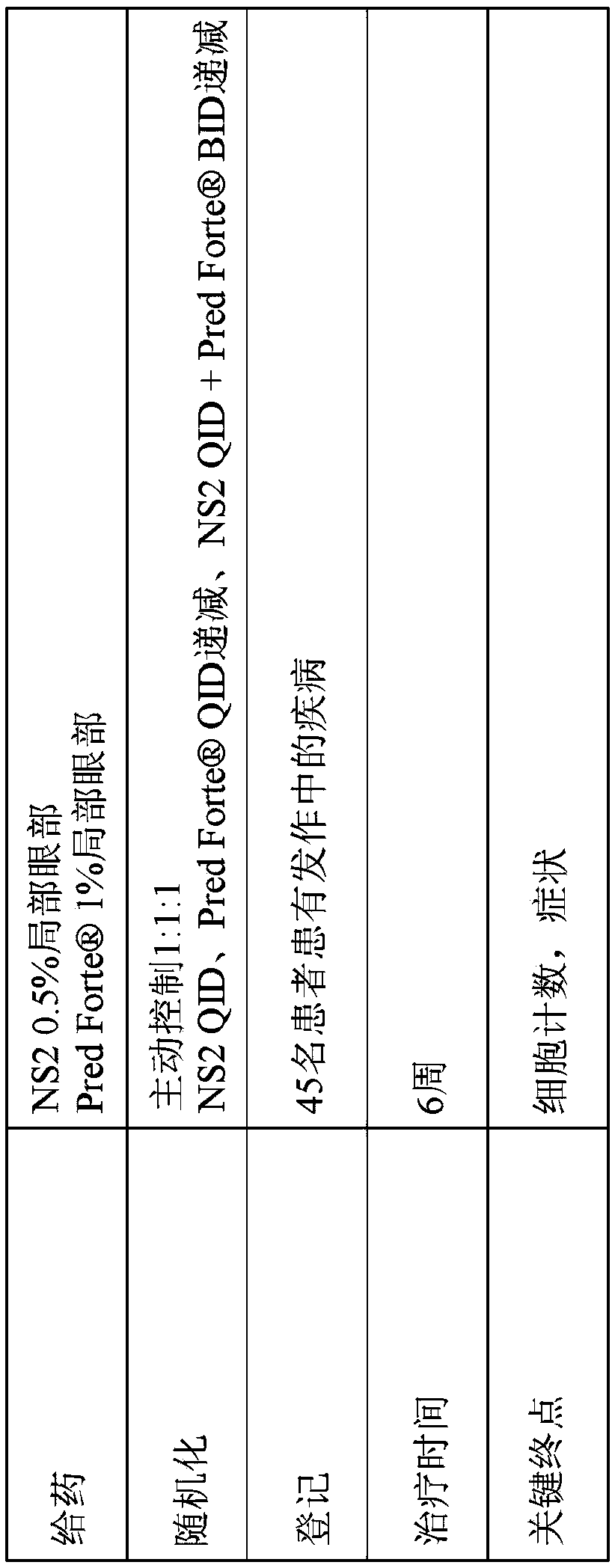
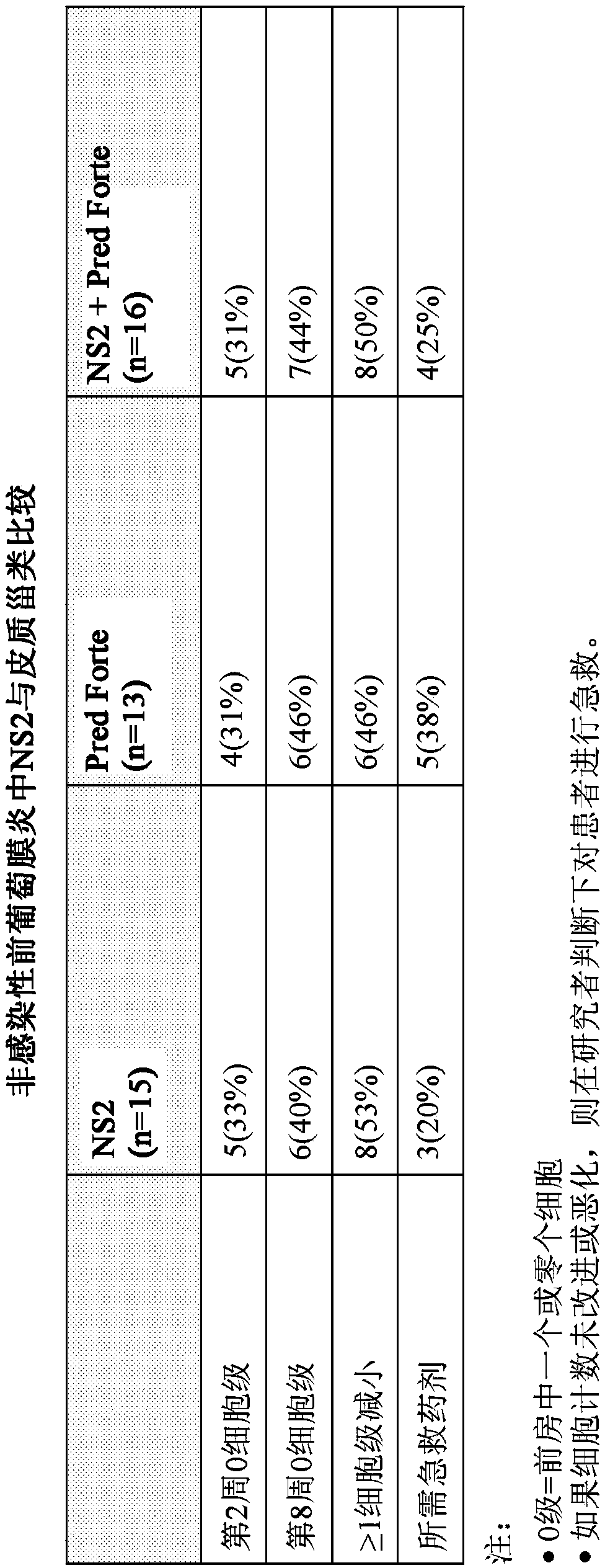
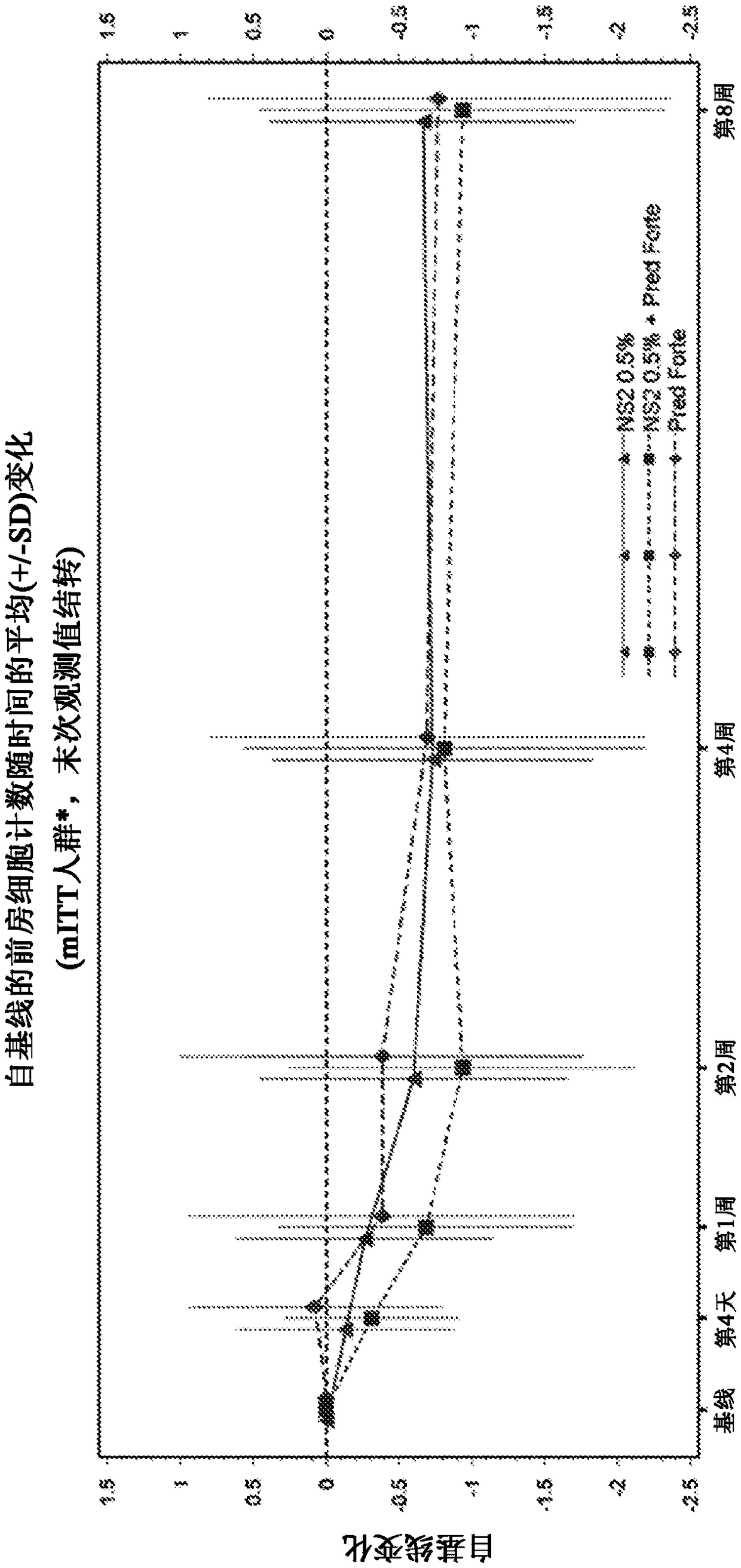
[0160] Example 1: Randomized, Investigator-masked comparator-controlled trial of NS2 compound eye drops in patients with anterior uveitis
[0161] Uveitis is inflammation of the eye involving structures in the uvea, such as the iris, ciliary body, and choroid. Specifically, anterior uveitis refers to uveitis in the anterior segment of the eye. It can also involve the posterior segment of the eye or the entire eye (panuveitis). Clinical signs and symptoms of anterior uveitis may include pain, blurred vision, conjunctival skin injection, anterior chamber cells, anterior chamber flare, fibrin deposits, and corneal endothelial inflammatory deposits. Uveitis is not a single disease entity, but a form of ocular inflammation associated with multiple groups of infectious and noninfectious conditions.
[0162] NS2 is a novel aldehyde scavenger formulated for this study as topical eye drops. Accumulation of toxic aldehyde metabolites such as malondialdehyde (MDA) is associated with a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap