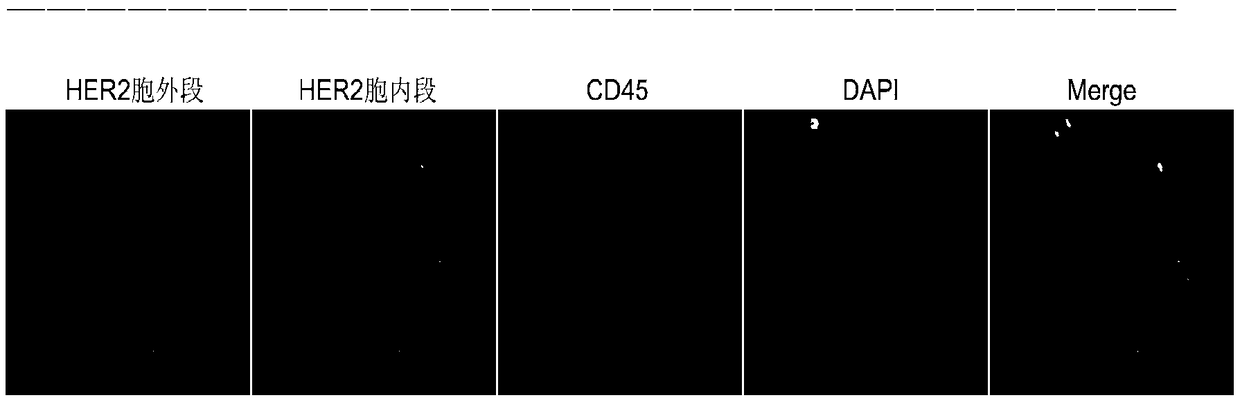
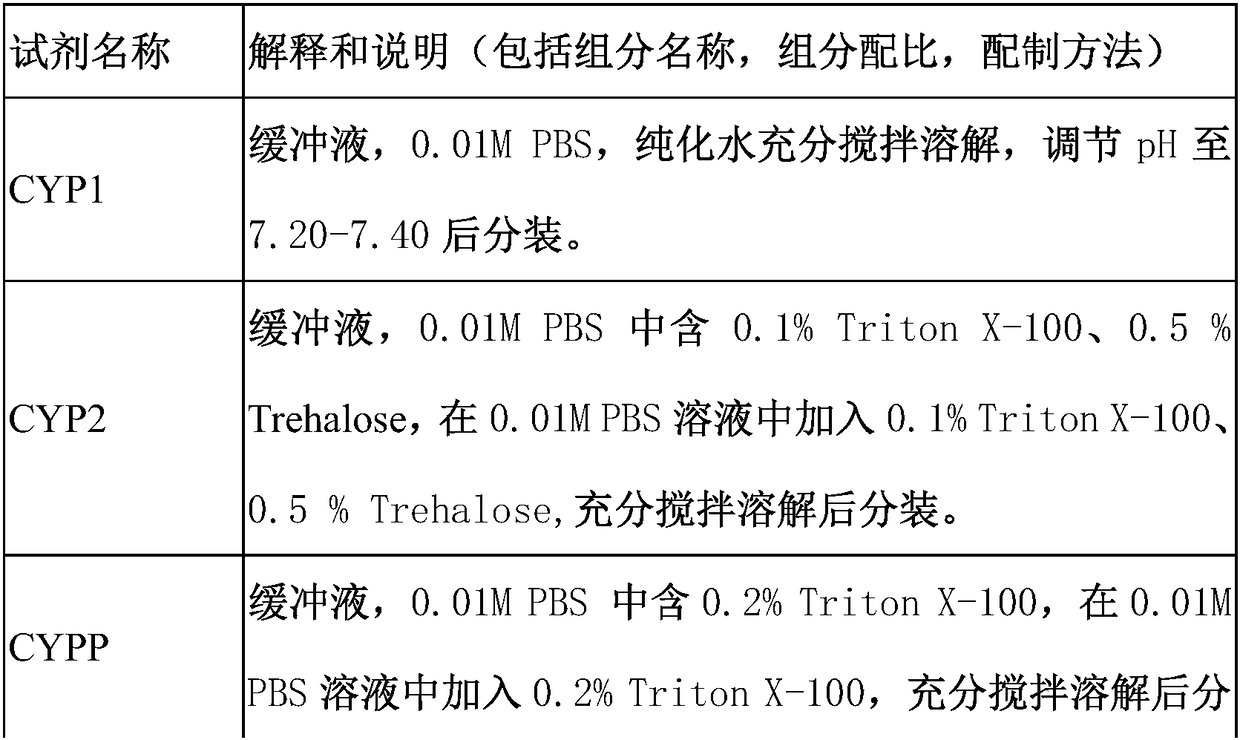
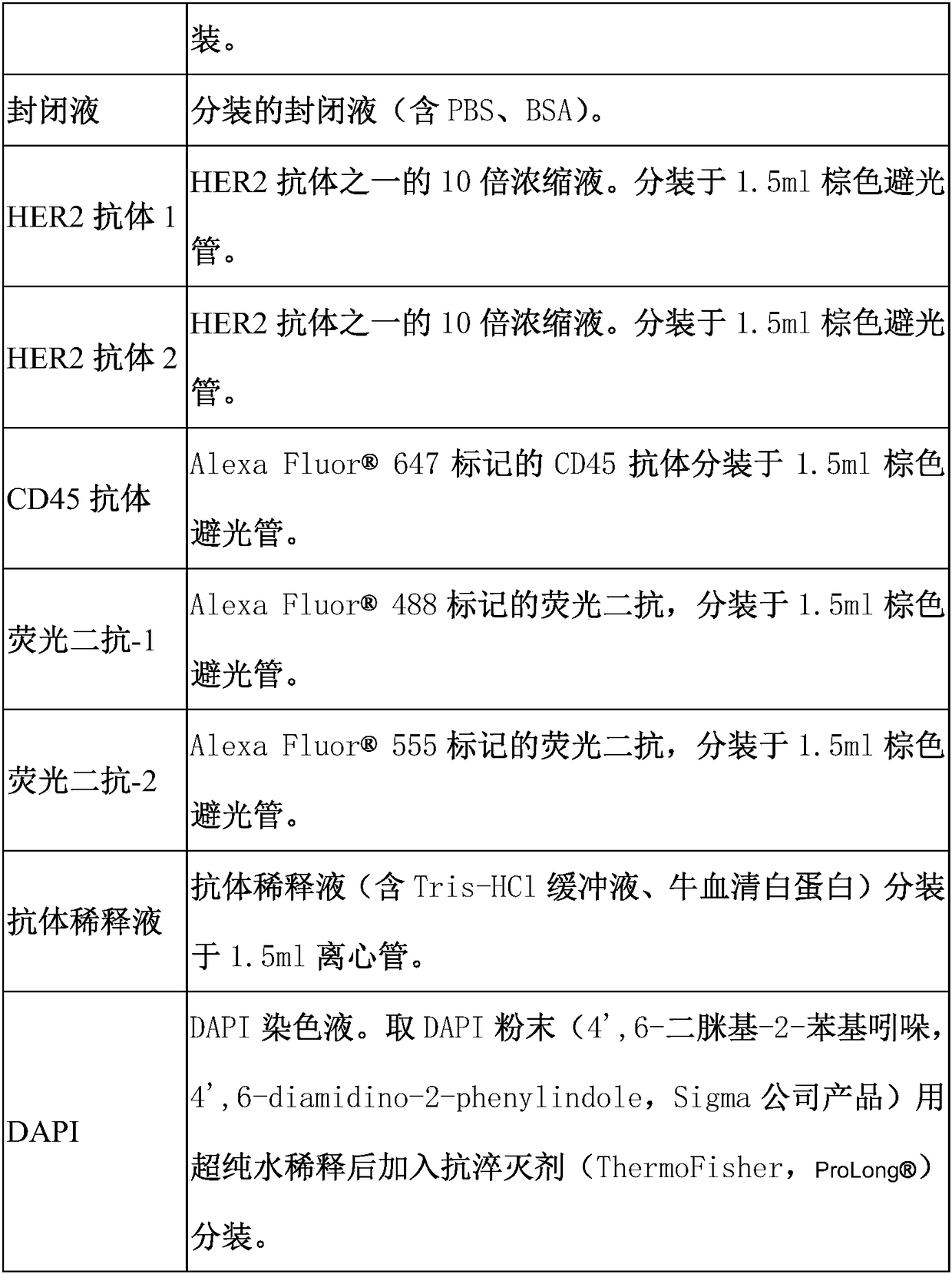
Immunofluorescent kit for detecting different loci of HER2 antigen and application of immunofluorescent kit
A technology of immunofluorescence detection and detection process, which is applied in the field of immunofluorescence detection kits for different sites of human epidermal growth factor receptor 2 antigen, which can solve the problems of inability to perform dynamic detection and inability to judge the absence of extracellular segments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Materials: smears of negatively enriched blood samples and SKBR3 smears for control cells.
[0080] Experimental steps:
[0081] 1. Draw 3.5ml of peripheral blood into an ACD (sodium citrate) anticoagulant tube. use The human peripheral blood leukocyte depletion kit negatively enriches tumor cells and fixes them on glass slides;
[0082] 2. Wash slides with CYP1 for 3 minutes x 3 times, 100-150 μL each time, to ensure that the entire sample area is covered;
[0083] 3. Absorb the excess liquid on the slide, add CYPP for 5 minutes, wash the slides with CYP1 as above for 3 minutes × 1 time; absorb excess liquid, add 200 μl of ice acetone:methanol (7:3) for 5 minutes, and wash the slides with CYP1 for 3 minutes × 3 times , to absorb excess water;
[0084] 4. Add 100-150 μl of blocking solution to block at room temperature for 25-30 minutes. Absorb excess blocking solution, add 100 μl of diluted HER2 antibody 1, HER2 antibody 2 and CD45 antibody, and incubate in a hum...
Embodiment 2
[0092] Materials: 1 tube of appropriate amount of anticoagulated blood, which is enriched by membrane filtration and then detected for protein. Experimental steps:
[0093] 1. Take an appropriate amount of peripheral blood and put it into a blood collection tube containing anticoagulant, and shake it slightly to mix.
[0094] 2. Add the suspension to the membrane filtration separation tumor cell technology device, and slowly pass through the filter and the filter membrane.
[0095] 3. After the filtration is completed, continue to add 50ml of 0.01M PBS to the membrane filtration device, wash the cell suspension attached around the tube wall into the membrane filtration device, and let it pass through the filter and membrane;
[0096] 4. Fix the cells on the filter membrane;
[0097] 5. Perform the same operation as in Example 1 to detect the protein.
Embodiment 3
[0099] Materials: 1 tube of appropriate amount of anticoagulated blood, which is enriched by microfluidic method and then detected for protein. Experimental steps:
[0100] 1. The appropriate amount of blood drawn is enriched using microfluidic chips of various principles.
[0101] 2. After enrichment, the samples were subjected to protein immunofluorescence detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com