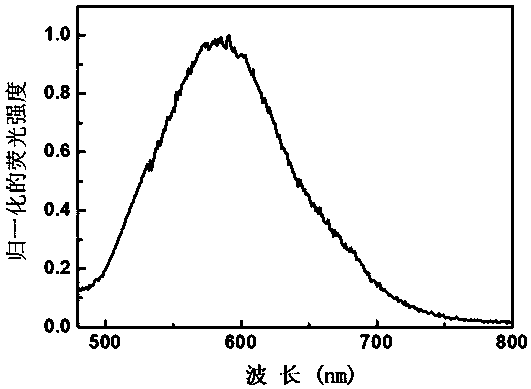
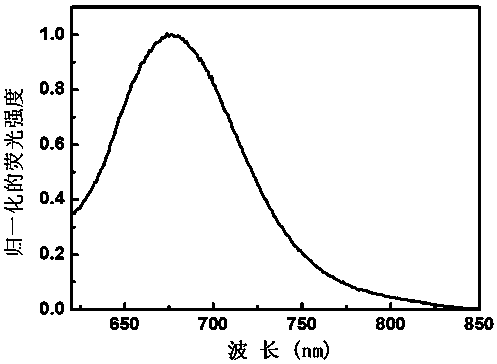
Fluorescent probe compound for detecting polysulfide and preparation method thereof
A polysulfide and fluorescent probe technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of complex operation methods and large instruments, and achieve good selectivity, simple detection operation, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for preparing a fluorescent probe compound (as shown in formula I) for polysulfide detection, the steps comprising:
[0035]
[0036] Formula I
[0037] (1) Preparation of Compound 1
[0038] 6-Bromo-2-naphthol (10g, 0.0426mol) was dissolved in 40ml of water, and methylamine hydrochloride (15g , 0.222mol) at 140°C for 96 hours. The reaction was cooled to room temperature, washed with aqueous sodium hydroxide solution (2mol / L), dried and recrystallized with methanol to obtain a brown-yellow product with a yield of 83.2%.
[0039] Compound one: 1 H NMR (500 MHz, CDCl 3 -D 1 ) δ (ppm): 2.84 (s, 3H), 3.79 (s,1H), 6.660-6.682 (d, 1H), 6.775-6.910 (q, 1H), 7.635-7.400 (q, 1H), 7.430-7.465 (d, 2H), 7.760-7.790 (d, 1H). 13 C NMR (125 MHz, CDCl 3 -D 1 ) δ (ppm): 147.28, 133.84, 129.65, 129.48, 128.53, 127.98, 127.69, 118.83, 114.98, 103.56, 30.68.
[0040]
[0041] compound one
[0042] (2) Preparation of Compound 2
[0043] Compound 1 (2g, 8.5mmol) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com