Preparation method of high-purity 5-acetylacetylaminobenzimidazolone
A technology of acetoacetamidobenzimidazolone and aminobenzimidazolone, which is applied in the field of preparation of high-purity 5-acetoacetamidobenzimidazolone, and can solve the problems of large environmental pollution, high production cost, poor safety, etc. problems, to achieve the effects of reducing environmental hazards, low production costs, and small environmental impacts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
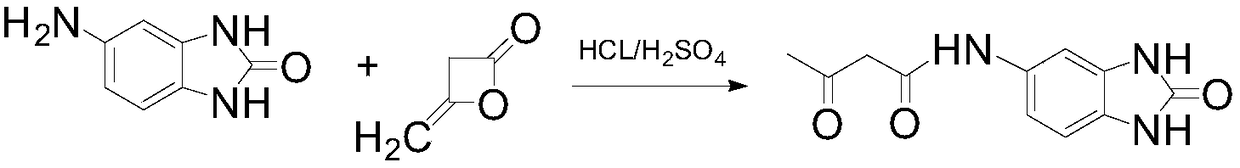
[0025] Add water 3700kg in reactor, wet weight is 51% 5-acetamidobenzimidazolone 285kg, sodium bisulfite 16kg, mass fraction is 20kg of hydrochloric acid of 30%, activated carbon 20kg starts to stir, is warming up to 68 ℃, reacts Press filter for 1 hour, heat up the filtrate to 60° C., add 20 kg of sodium hydroxide and 108 kg of diketene to react for 4 hours, cool down and press filter to obtain 5-acetoacetamido benzimidazolone.
[0026] The 5-acetoacetamidobenzimidazolone obtained by the above examples of the present invention is detected by liquid chromatography, the purity of the 5-acetoacetamidobenzimidazolone prepared by the present invention is 99.5013%, the raw material peak is 0.0152%, and the acetic anhydride peak is 0.1506%, in line with industry standards.
[0027] The 5-acetoacetamidobenzimidazolone obtained in the above example of the present invention is detected, and the yield of the 5-acetoacetamidobenzimidazolone prepared in the present invention is 88.13%.
Embodiment 2
[0029] Add water 3700kg in the reactor, wet weight is 50% 5-acetamidobenzimidazolone 285kg, sodium bisulfite 16kg, mass fraction is 30% sulfuric acid 10kg, gac 20kg starts to stir, is warming up to 65 ℃, reacts Press filter for 1 hour, heat up the filtrate to 60° C., add 20 kg of sodium hydroxide and 108 kg of diketene to react for 4 hours, cool down and press filter to obtain 5-acetoacetamido benzimidazolone.
[0030] The 5-acetoacetamidobenzimidazolone obtained by the above examples of the present invention is detected by liquid chromatography, the purity of the 5-acetoacetamidobenzimidazolone prepared by the present invention is 99.6196%, the raw material peak is 0.0110%, and the acetic anhydride peak is 0.1769%, in line with industry standards.
[0031] The 5-acetoacetamidobenzimidazolone obtained in the above example of the present invention was detected, and the yield of the 5-acetoacetamidobenzimidazolone prepared in the present invention was 86.52%.
Embodiment 3
[0033] Add 3700kg of water in the reactor, 280kg of 5-acetamidobenzimidazolone with a wet weight of 48%, 15kg of sodium bisulfite, 22kg of hydrochloric acid with a mass fraction of 30%, and 20kg of activated carbon to start stirring, heat up to 66°C, and react Press filter for 1 hour, heat up the filtrate to 60° C., add 22 kg of sodium hydroxide and 108 kg of diketene to react for 4 hours, cool down and press filter to obtain 5-acetoacetamido benzimidazolone.
[0034] The 5-acetoacetamidobenzimidazolone obtained by the above examples of the present invention is detected by liquid chromatography, the purity of the 5-acetoacetamidobenzimidazolone prepared by the present invention is 99.6351%, the raw material peak is 0.0156%, and the acetic anhydride peak is 0.0888%, in line with industry standards.
[0035] The 5-acetoacetamidobenzimidazolone obtained in the above example of the present invention is detected, and the yield of the 5-acetoacetamidobenzimidazolone prepared in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com