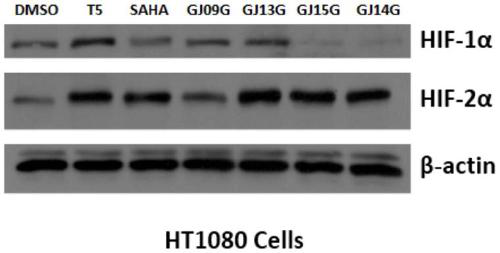
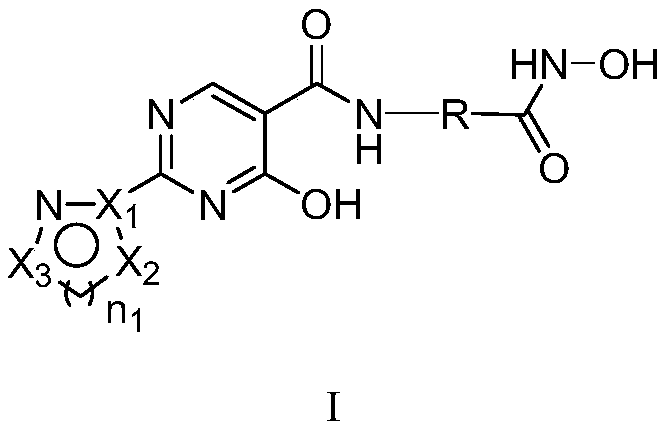
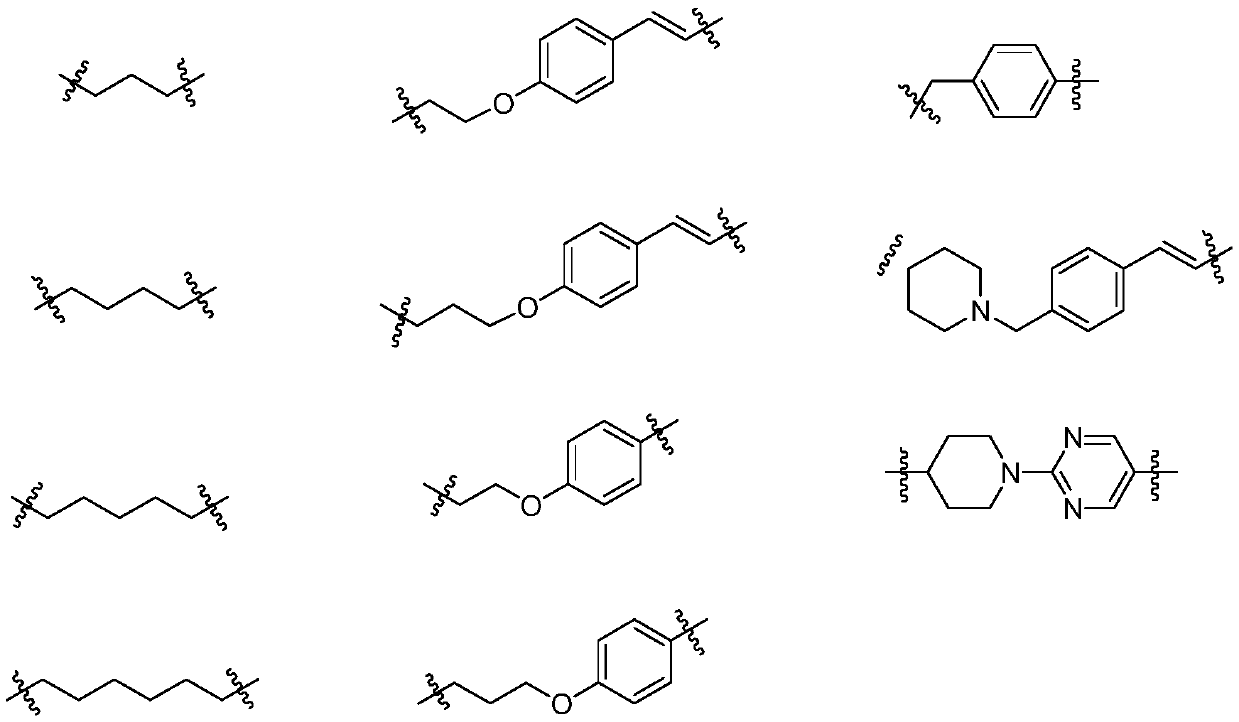
Dual inhibitor of prolyl hydroxylase and histone deacetylase, preparation method and application thereof
A reaction and compound technology, applied in the application field of preparing radiation protection drugs, can solve the problem of lack of similar compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 4-Hydroxy-2-pyrazol-1-ylpyrimidine-5-carboxylic acid ethyl ester (2)
[0061]
[0062] Add pyrazole-1-carboxamide hydrochloride (1eq) and N,N-diisopropylethylamine (2.2eq) to 5 times the volume of ethanol, stir at room temperature for 20min, add ethoxymethylenemalonate diethyl Ester (1eq), warmed to reflux and reacted overnight. After the reaction was complete, about half of the volume of ethanol was evaporated under reduced pressure, water and ethyl acetate were added to extract twice, the organic layer was washed once with water, and the aqueous layers were combined. The pH of the aqueous layer was adjusted to 3-4, and a large amount of white insoluble matter was precipitated, filtered and dried to obtain a white powder of 2 with a yield of about 90.25%. m.p.162.8-163.8℃; ESI-MS(m / z):235.0822[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 )δ13.28(s,1H),8.63–8.56(m,2H),7.96(dd,J=1.6,0.7Hz,1H),6.67(dd,J=2.8,1.6Hz,1H),4.26(q ,J=7.1Hz,2H),1.29(t,J=7.1Hz,3H)
Embodiment 2
[0063] Example 2 4-(4-methoxybenzyloxy)-2-pyrazol-1-ylpyrimidine-5-carboxylic acid ethyl ester (3)
[0064]
[0065] Add 2 and anhydrous potassium carbonate (1.4eq) to 5 times the volume of N,N-dimethylformamide, stir at room temperature for 30min, add a catalytic amount of potassium iodide (0.1eq), and then p-methoxychloride Benzyl chloride was dissolved in 5 times the volume of N,N-dimethylformamide, added dropwise to the system, heated to 80°C, and reacted overnight. TLC monitoring, after the reaction is complete, add 10 times the volume of water to the system, then extract twice with ethyl acetate, combine the organic layers, and then use saturated NaHCO 3 The solution was washed twice, and the organic layer was dried over anhydrous sodium sulfate. After removing the solvent under reduced pressure, the residue was pulped with methanol, and the filter cake was washed with the mother liquor. The white solid powder of 3 was obtained after the filter cake was dried, and t...
Embodiment 3
[0066] Example 3 4-(4-methoxybenzyloxy)-2-pyrazol-1-ylpyrimidine-5-carboxylic acid ethyl ester (4)
[0067]
[0068]3 and sodium hydroxide (5eq) were dissolved in 10 volumes of tetrahydrofuran / water mixed solvent (volume ratio 1:1), stirred at room temperature, and the reaction was monitored by TLC. After the reaction was complete, part of the tetrahydrofuran was distilled off under reduced pressure, extracted once by adding water and ethyl acetate, and the pH of the aqueous layer was adjusted to 3-4 to precipitate a white solid, which was filtered and dried to obtain a white powder of 4 with a yield of about 87.97%. m.p.126.3-126.5℃; ESI-MS(m / z):327.3[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 )δ13.17(s,1H),8.95(s,1H),8.74(d,J=2.7Hz,1H),7.93(d,J=1.4Hz,1H),7.50(d,J=8.6Hz, 2H), 6.93(d, J=8.6Hz, 2H), 6.64(dd, J=2.8, 1.6Hz, 1H), 5.57(s, 2H), 3.75(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com