Long-acting hypoglycemic weight-reducing peptide as well as preparation method and application thereof as medicine
A technology for hypoglycemic peptides and drugs, applied in the field of its preparation, long-acting hypoglycemic and weight-reducing peptides, can solve the problems of short half-life, weak hypoglycemic activity, etc., achieve short synthesis cycle, long drug effect time, and convenient purification work Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
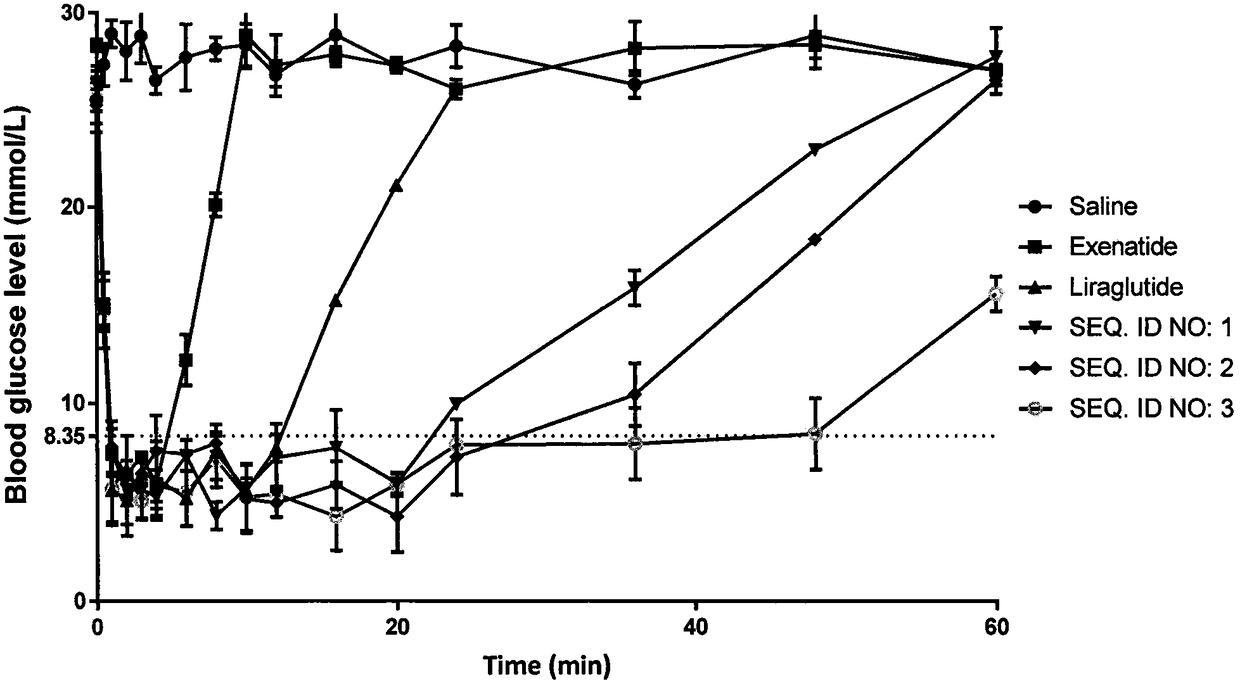
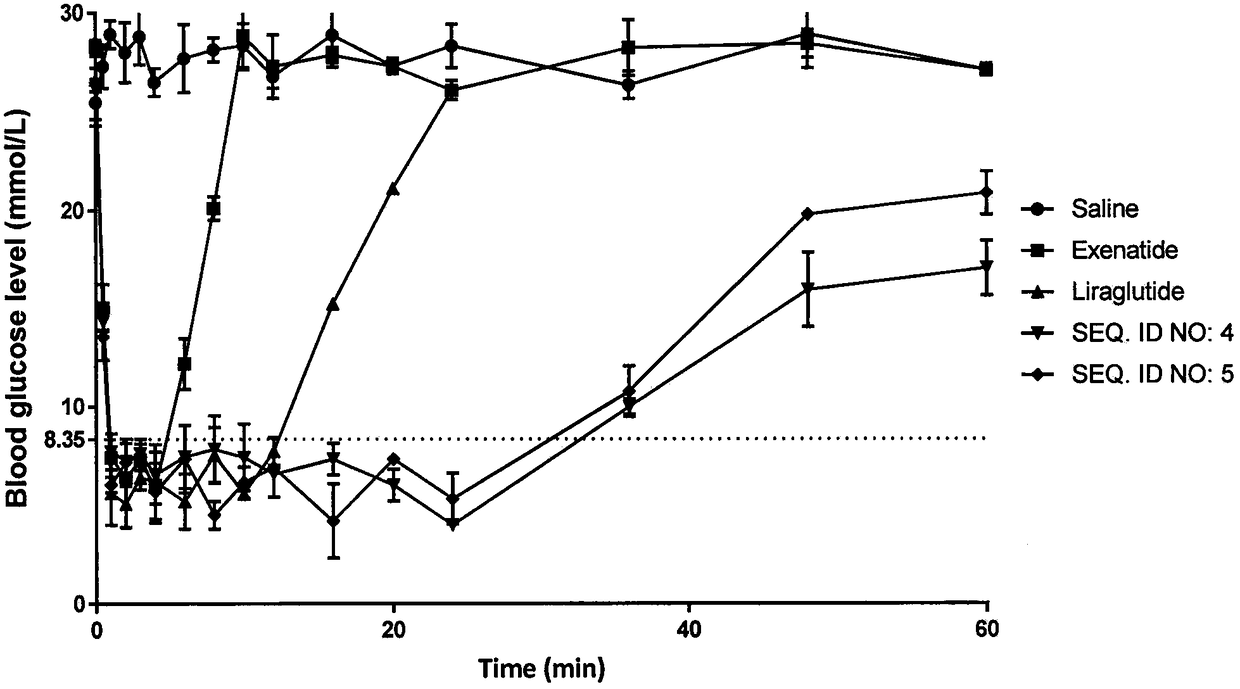
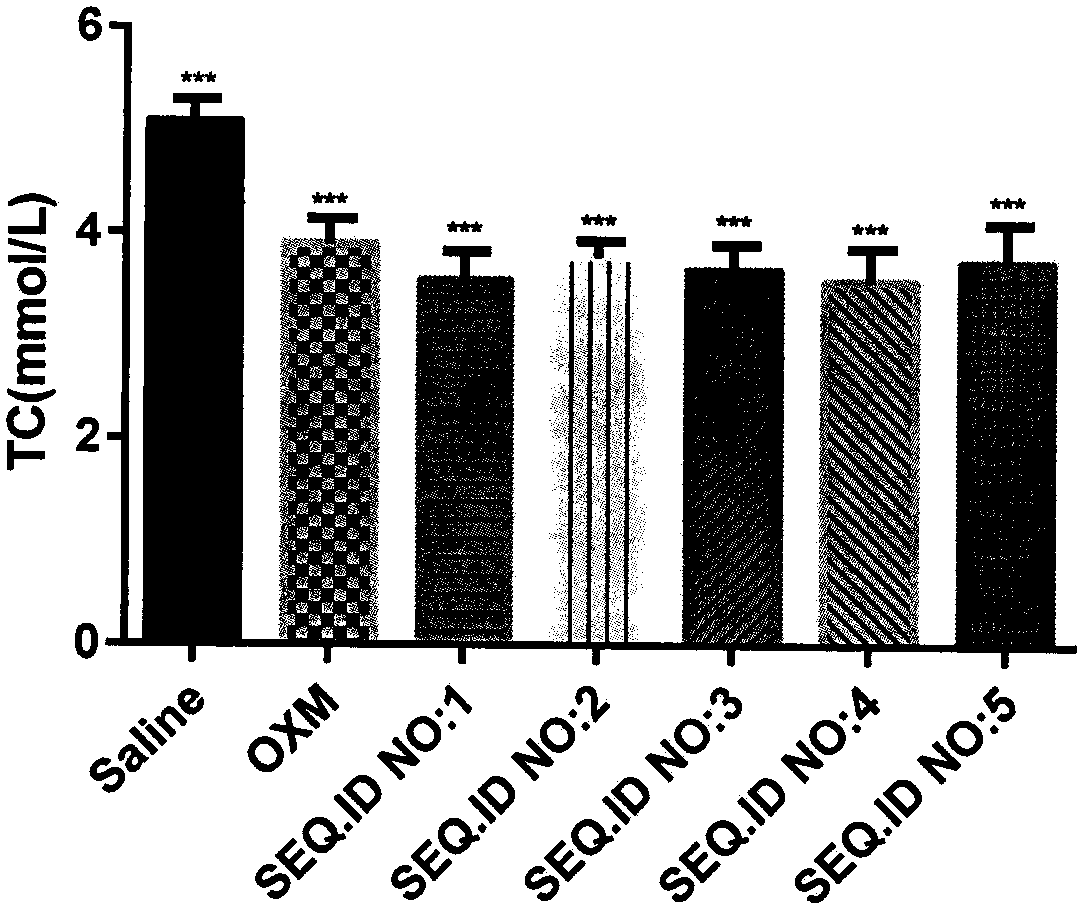
Image
Examples
Embodiment 1
[0060]
[0061] solid phase synthesis
[0062] 1. Synthesis of peptide chains
[0063] 1.1 Resin swelling
[0064] Weigh 50mg of Fmoc-Rink amide-MBHA Resin (degree of substitution: 0.4mmol / g), swell with 7mL of DCM for 30min, filter to remove DCM, then swell with 10mL of NMP for 30min, rinse with NMP and 7mL of DCM respectively.
[0065] 1.2 Removal of Fmoc protecting group
[0066] Put the swollen resin into the reactor, add 25% piperidine / NMP (V / V) solution containing 0.1M HOBt to the resin to remove Fmoc, and wash it with NMP after the reaction. A resin free of the initially attached Fmoc protecting group is obtained.
[0067] 1.3 Synthesis of Fmoc-Ser(tBu)-Rink amide-MBHA Resin
[0068] Fmoc-Ser(tBu)-OH (15.4 mg, 0.04 mmol), HBTU (15.1 mg, 0.04 mmol), HOBt (5.4 mg, 0.04 mmol) and DIPEA (13.9 μL, 0.08 mmol) were dissolved in NMP 10 mL, and then This solution was added to the resin obtained in the previous step, and reacted for 2 hours. After completion, the reaction...
Embodiment 2
[0079]
[0080] 1. Synthesis of 16-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)hexadecanoic acid
[0081] 16-Aminohexadecanic acid (1.09g, 4mmol) and maleic anhydride (0.47g, 4.8mmol) were dissolved in glacial acetic acid, and after ultrasonic dissolution, they were refluxed at 120°C for 6h. After the reaction was completed by TLC, the reaction The liquid was cooled to room temperature, extracted three times with ethyl acetate (3×20mL), the upper layer extracts were combined, and the extracts were washed three times with saturated brine, anhydrous Na 2 SO 4 Let dry overnight. The extract was spin-dried in vacuo to obtain a crude product, which was separated by column chromatography (ethyl acetate / petroleum ether) to obtain 1.02 g of a light yellow pure product with a yield of 72%.
[0082] 1 H-NMR (DMSO-d 6 , 300MHz): δppm: 12.45(s, 1H, -COO H ), 7.50 (s, 2H, -COC H =C H CO-), 3.88(t, 2H, J=7.0Hz, -NC H 2 - ), 2.68(t, J=7.3Hz, 2H, -C H 2 COOH), 2.00-1.96 (m, 4H, -NCH 2...
Embodiment 3
[0086]
[0087] 1. Synthesis of chemical modification groups
[0088] Synthesis of 3,3'-(4-carboxybenzylidene)-di-4-hydroxycoumarin
[0089] p-Carboxybenzaldehyde (0.45g, 3mmol) was dissolved in 20ml of absolute ethanol, and then 4-hydroxycoumarin (0.98g, 6mmol) was added. After heating to reflux for 12 hours, the reaction solution was cooled to room temperature and then filtered, and the filter cake was washed 3 times with 10ml of ethanol to obtain 1.12g of the product, with a yield of 82.1%, ESI-MS m / z: 456.4[M+H] + .
[0090] Synthesis of tert-butyl (12-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)dodecyl)carbamate
[0091] Dissolve N-Boc-dodecyldiamine (1.2g, 4mmol) and maleic anhydride (0.49g, 4.8mmol) in glacial acetic acid, heat the reaction at 120°C for 6h, after the TLC detection reaction is complete, the reaction The liquid was cooled to room temperature, extracted with ethyl acetate (3×20mL), the upper layer extracts were combined, and the extracts were washed 3 time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com