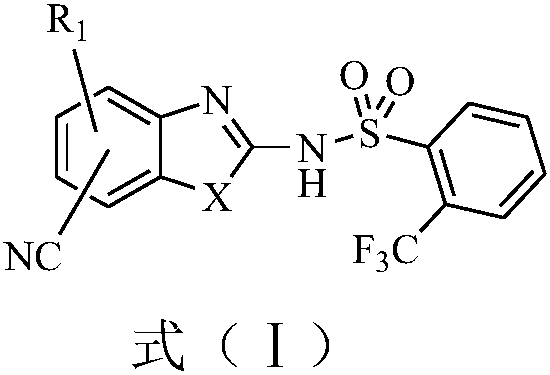
Medical application of 2-(trifluoromethyl)benzenesulfonamide derivatives
A use and pharmaceutical technology, applied in the field of medical use of 2-trifluoromethylbenzenesulfonamide derivatives, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
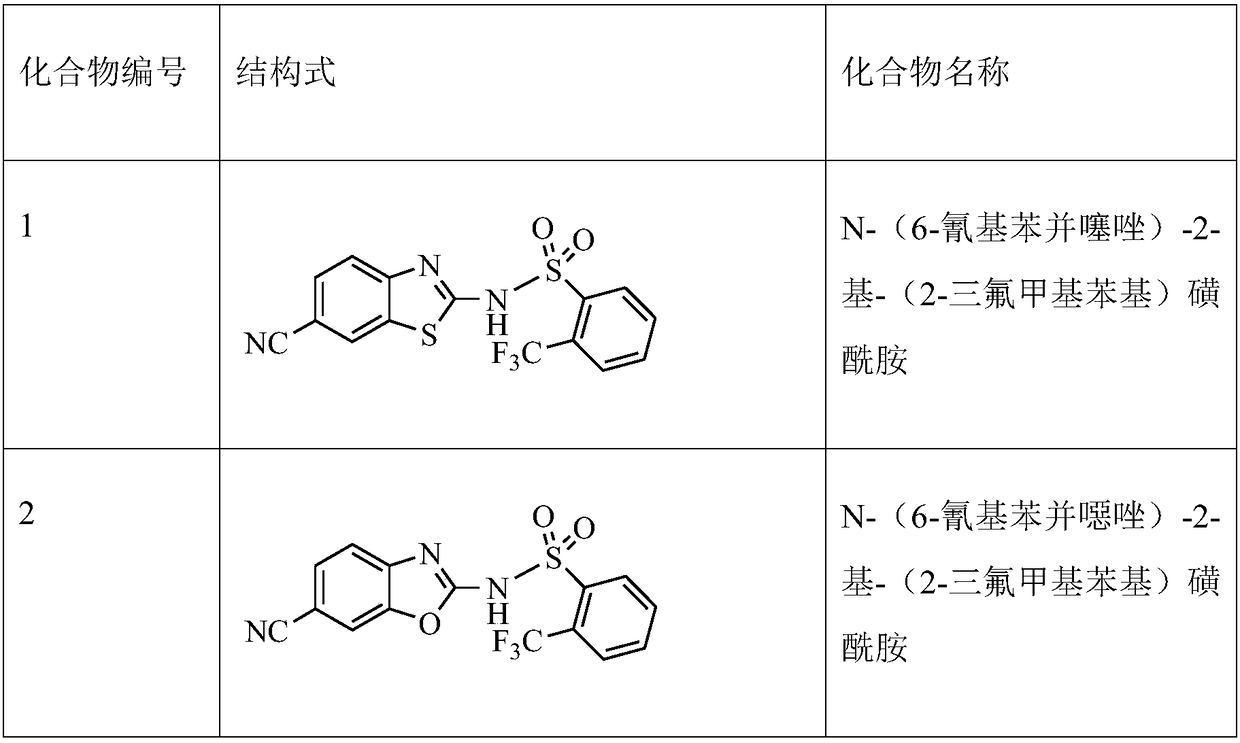
[0030] Compound 2-amino-6-cyanobenzothiazole 1-A (700mg, 4mmol) was dissolved in 7ml of anhydrous THF, cooled to about -20°C, and LDA / THF solution (4.8mL, 1mmol / ml, 1.2eq), after the addition, continue to stir at this temperature for 0.5h, add 2-trifluoromethylbenzene-1sulfonyl chloride (1.17g, 4.8mmol, 1.2eq), after the addition, continue to stir at 0°C for 2 hours . Extracted with ethyl acetate, the crude product was separated and purified by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate (V / V) = 1:3, to obtain compound 1 (1.2 g, yield 82%).
[0031] MS m / z(ESI):384[M+1]; 1 HNMR (400MHz, d-DMSO) 13.9 (s, br, 1H), 8.27-8.35 (m, 2H), 7.83-7.97 (m, 4H), 7.43 (d, J=7.2Hz, 1H).
Embodiment 2
[0033]
[0034] The compound 2-amino-6-cyanobenzoxazole 2-A (636mg, 4mmol) was dissolved in 7ml of anhydrous THF, cooled to about -20°C, and LDA / THF solution (4.8mL, 1mmol / ml , 1.2eq), continue stirring at this temperature for 0.5h after addition, add 2-trifluoromethylbenzene-1sulfonyl chloride (1.17g, 4.8mmol, 1.2eq), continue stirring at 0°C for 2 Hour. Extracted with ethyl acetate, the crude product was separated and purified by silica gel column chromatography, the eluent was petroleum ether:ethyl acetate (V / V)=1:3, to obtain compound 2 (1.15g, yield 78%).
[0035] MS m / z(ESI):368[M+1]; 1 HNMR (400MHz, d-DMSO) 8.34 (d, J = 8.0Hz, 1H), 8.10 (s, 1H), 7.96 (d, J = 8.0Hz, 1H), 7.82-7.91 (m, 2H), 7.74 ( d,J=8.0Hz,1H),7.43(d,J=8.0Hz,1H).
Embodiment 3
[0037]
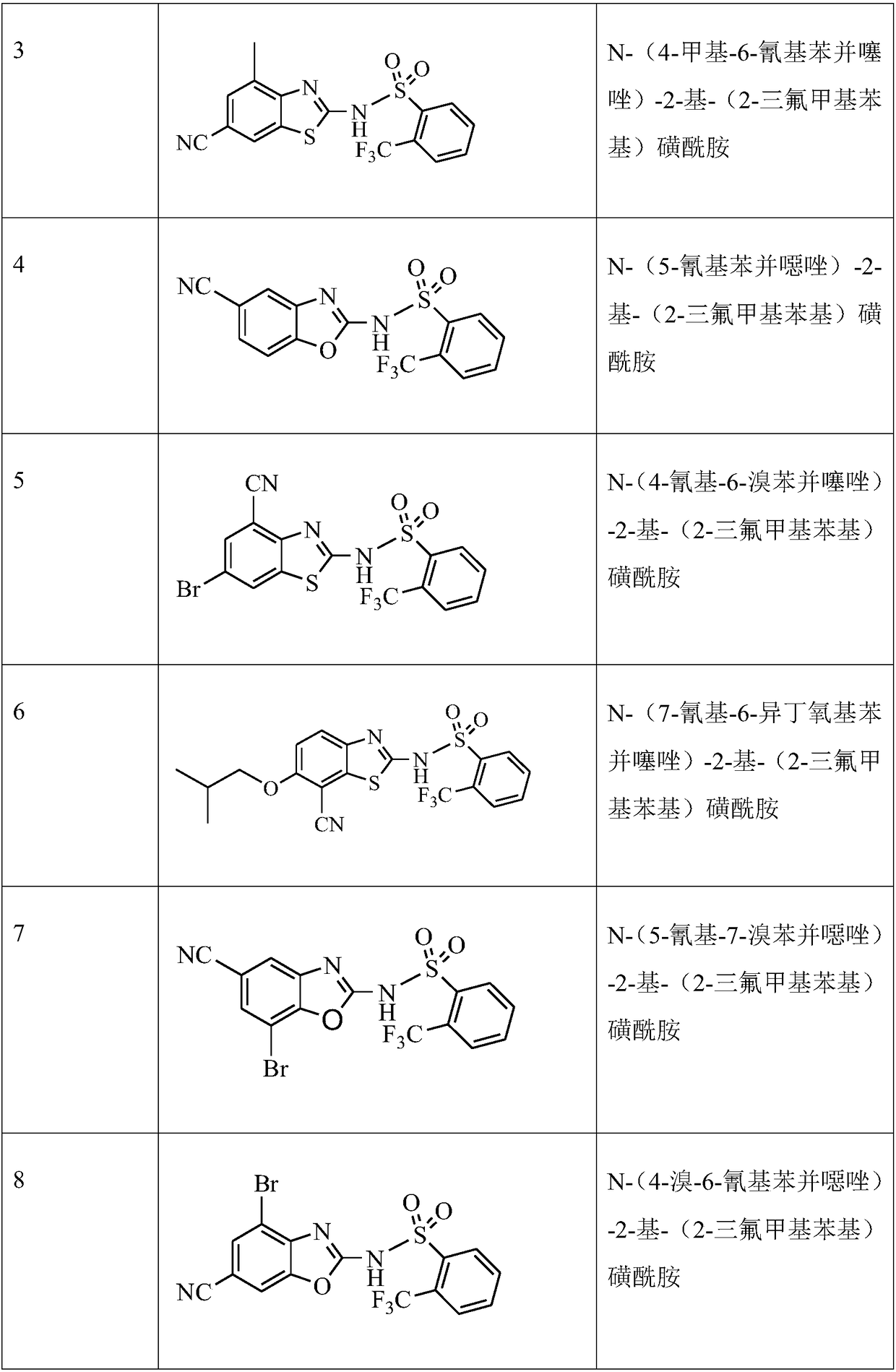
[0038] The compound 2-amino-4-methyl-6-cyanobenzothiazole 3-A (756mg, 4mmol) was dissolved in 8ml of anhydrous THF, cooled to about -20°C, and LDA / THF solution (4.8mL , 1mmol / ml, 1.2eq), after the addition, continue to stir at this temperature for 0.5h, add 2-trifluoromethylbenzene-1 sulfonyl chloride (1.17g, 4.8mmol, 1.2eq), after the addition, at 0 ℃ Stirring was continued for 2 hours. Extracted with ethyl acetate, the crude product was separated and purified by silica gel column chromatography, the eluent was petroleum ether:ethyl acetate (V / V)=1:2, to obtain compound 3 (1.25g, yield 79%).
[0039] MS m / z(ESI):398[M+1]; 1 HNMR (400MHz, d-DMSO) 13.76 (s, br, 1H), 8.28 (d, J = 8.0Hz, 1H), 8.20 (s, 1H), 7.99 (d, J = 8.0Hz, 1H), 7.86- 7.91(m,2H),7.70(s,1H),2.43(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com