Application of p-tolylanilide Lithium in Catalytic Hydroboration of Imine and Borane
A technology based on methylaniline and catalytic imine, which is applied in the direction of chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. long time, expensive catalyst, etc., to achieve the effect of wide substrate application range, short reaction time and good universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
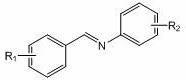
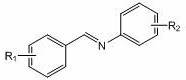
[0024] Example 1: Lithium p-methylanilide catalyzes the hydroboration reaction of benzidine and pinacol borane
[0025] In the reaction flask that has been dehydrated and deoxygenated, add 0.5 mmol of benzylaniline under the protection of argon, add 100ul THF, then add 0.5 mmol (0.0726 mL) borane with a pipette and mix well, and finally add 34.4 ul of p-methyl Tetrahydrofuran solution (0.7273M) of lithium anilide (5 mol% dosage, the same below), after reacting for 2 h, draw a drop into the NMR tube with a dropper, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 91%. NMR data of the product: 1 H NMR (CDCl 3 ,400 MHz) δ: 7.29~7.12(m, 9H), 6.88~6.84 (t, 1H), 4.69 (s, 2H), 1.29 (s, 12H).
Embodiment 2
[0026] Embodiment two: p-methylanilide lithium catalyzes the hydroboration reaction of benzidine and pinacol borane
[0027] In the reaction flask that has been dehydrated and deoxygenated, add 0.5 mmol of benzylaniline under the protection of argon, add 100ul THF, then add 0.6 mmol (0.0871 mL) borane with a pipette and mix well, and finally add 34.4 ul of p-methyl Tetrahydrofuran solution (0.7273M) of lithium anilide (0.7273M) (5 mol% dosage, the same below), after reacting for 1 h, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 96%. NMR data of the product: 1 H NMR (CDCl 3 , 400MHz) δ : 7.29~7.12(m, 9H), 6.88~6.84 (t, 1H), 4.69 (s, 1H), 1.29 (s, 12H).
Embodiment 3
[0028] Example 3: Lithium p-methylanilide catalyzes the hydroboration reaction of benzidine and pinacol borane
[0029] In the reaction flask that has been dehydrated and deoxygenated, add 0.5 mmol of benzylaniline under the protection of argon, add 100ul THF, then add 0.6 mmol (0.0871 mL) borane with a pipette and mix well, and finally add 34.4 ul of p-methyl Tetrahydrofuran solution (0.7273M) of lithium anilide (5 mol% dosage), after reacting for 2 h, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (CDCl 3 , 400MHz) δ : 7.29~7.12(m, 9H), 6.88~6.84 (t, 1H), 4.69 (s, 2H), 1.29 (s, 12H).
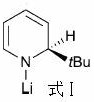
[0030] Substitution of p-toluidelithium by the amide lithium compound of formula I did not yield the product.
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com