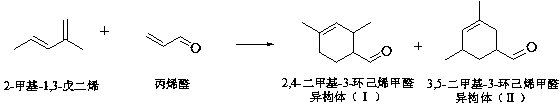Method for continuous synthesis of ligustral in fixed bed reactor
A technology of fixed bed reactor and privet aldehyde, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve problems such as reaction temperature, high pressure, acrolein polymerization, difficult temperature control, etc. Achieve the effect of avoiding polymerization, low pressure and avoiding violent reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The method for continuously synthesizing privetal in a kind of fixed-bed reactor of embodiment 1
[0040] Include the following steps:
[0041] (1) Mixing of raw materials
[0042] 165.1g (2.01mol) 2-methyl-1,3-pentadiene and 114.9g (2.05mol) acrolein are mixed, and the molar ratio of 2-methyl-1,3-pentadiene and acrolein in the mixture is 1:1.02, put the mixed solution in the raw material solution 1 storage tank.
[0043] (2) Air replacement, preheating
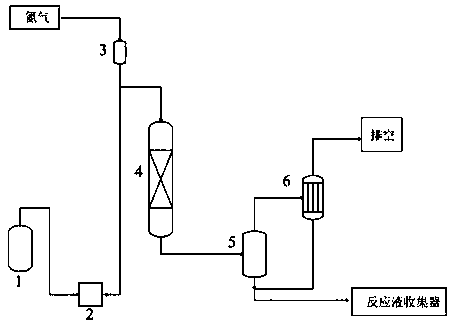
[0044]The fixed-bed reactor 4 passes nitrogen from the nitrogen buffer tank 3 to replace the air, and the temperature is raised for preheating.
[0045] (3) Continuous response
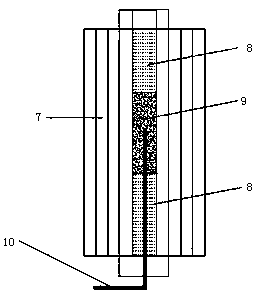
[0046] When the temperature of the catalyst bed in the reactor reaches the reaction temperature, feed the raw material liquid 1 into the reactor through the double-plunger micrometering pump 2, and fill the fixed bed reactor 4 with phosphomolybdic acid modified Al 2 o 3 Catalyst, catalyst loading is 15g, reaction temperature is 36°C, rea...
Embodiment 2
[0049] The method for continuously synthesizing privetal in a kind of fixed-bed reactor of embodiment 2
[0050] Include the following steps:
[0051] (1) Mixing of raw materials
[0052] 165.1g (2.01mol) 2-methyl-1,3-pentadiene and 129.6g (2.31mol) acrolein are mixed, and the molar ratio of 2-methyl-1,3-pentadiene and acrolein in the mixture is 1:1.15, put the mixed solution in the raw material solution 1 storage tank.
[0053] (2) Air replacement, preheating
[0054] The fixed-bed reactor 4 passes nitrogen from the nitrogen buffer tank 3 to replace the air, and the temperature is raised for preheating.
[0055] (3) Continuous response
[0056] When the temperature of the catalyst bed in the reactor reaches the reaction temperature, feed the raw material liquid 1 into the reactor through the double-plunger micrometering pump 2, and fill the fixed bed reactor 4 with phosphomolybdic acid modified Al 2 o 3 Catalyst, catalyst loading is 20g, reaction temperature is 40°C, re...
Embodiment 3
[0059] The method for continuously synthesizing privetal in a kind of fixed-bed reactor of embodiment 3
[0060] Include the following steps:
[0061] (1) Mixing of raw materials
[0062] 165.1g (2.01mol) 2-methyl-1,3-pentadiene and 123.9g (2.21mol) acrolein are mixed, and the molar ratio of 2-methyl-1,3-pentadiene and acrolein in the mixture is 1:1.10, put the mixed solution in the raw material solution 1 storage tank.
[0063] (2) Air replacement, preheating
[0064] The fixed-bed reactor 4 passes nitrogen from the nitrogen buffer tank 3 to replace the air, and the temperature is raised for preheating.
[0065] (3) Continuous response
[0066] When the temperature of the catalyst bed in the reactor reaches the reaction temperature, feed the raw material liquid 1 into the reactor through the double plunger micro metering pump 2, and fill the fixed bed reactor 4 with phosphotungstic acid modified Al 2 o 3 Catalyst, catalyst loading is 20g, reaction temperature is 40°C, r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com