Method for building renal anemia animal model
A technology of animal model and construction method, which is applied in the field of medical engineering, and can solve problems such as models not being commonly used, lack of animal models of renal anemia, and difficult standardization of surgery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 renal anemia mouse model
[0034] In the first step, aristolochic acid was prepared into a 0.4 mg / mL solution with PBS.
[0035] In the second step, the C57BL / 6 mice were allowed to eat and drink freely for a week for adaptive feeding, and then the mice were divided into groups of 10, divided into 3 groups, and a group of mice without injection was used as the control group (referred to as the control group). con group), a group of mice were injected with aristolochic acid solution every 2 days (called AA / 2d group), and a group of mice were injected with aristolochic acid solution every 3 days (called AA / 3d group).
[0036] The mice in the AA / 2d group were injected intraperitoneally with aristolochic acid solution, and the injection volume and frequency were: 3 mg / kg, once every 2 days, for 6 consecutive weeks.
[0037] The mice in the AA / 3d group were intraperitoneally injected with aristolochic acid solution, the injection volume and fre...
Embodiment 2
[0038] Embodiment 2 detects each index of mouse
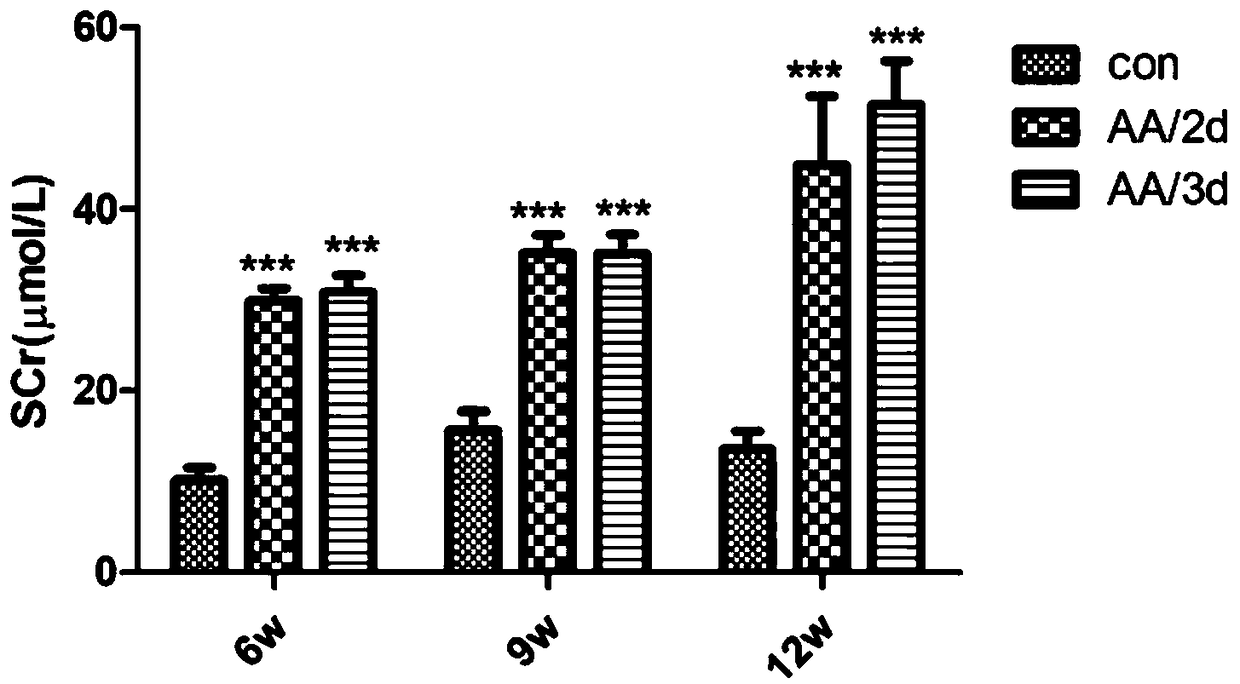
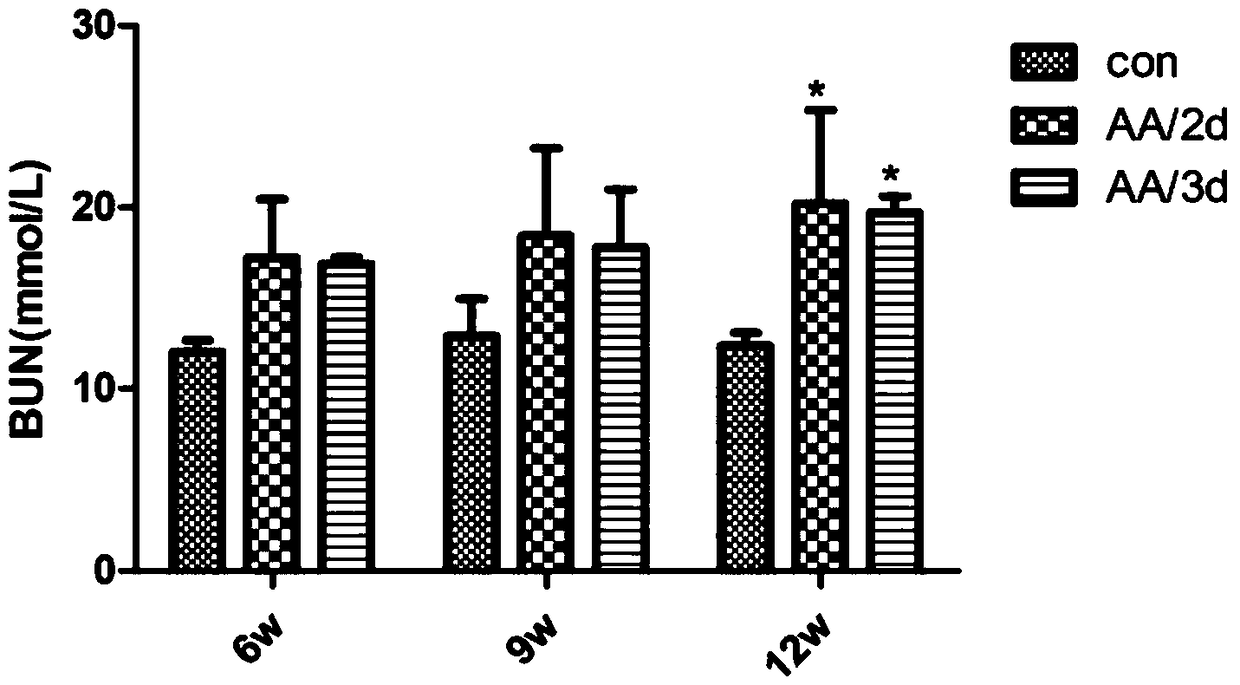
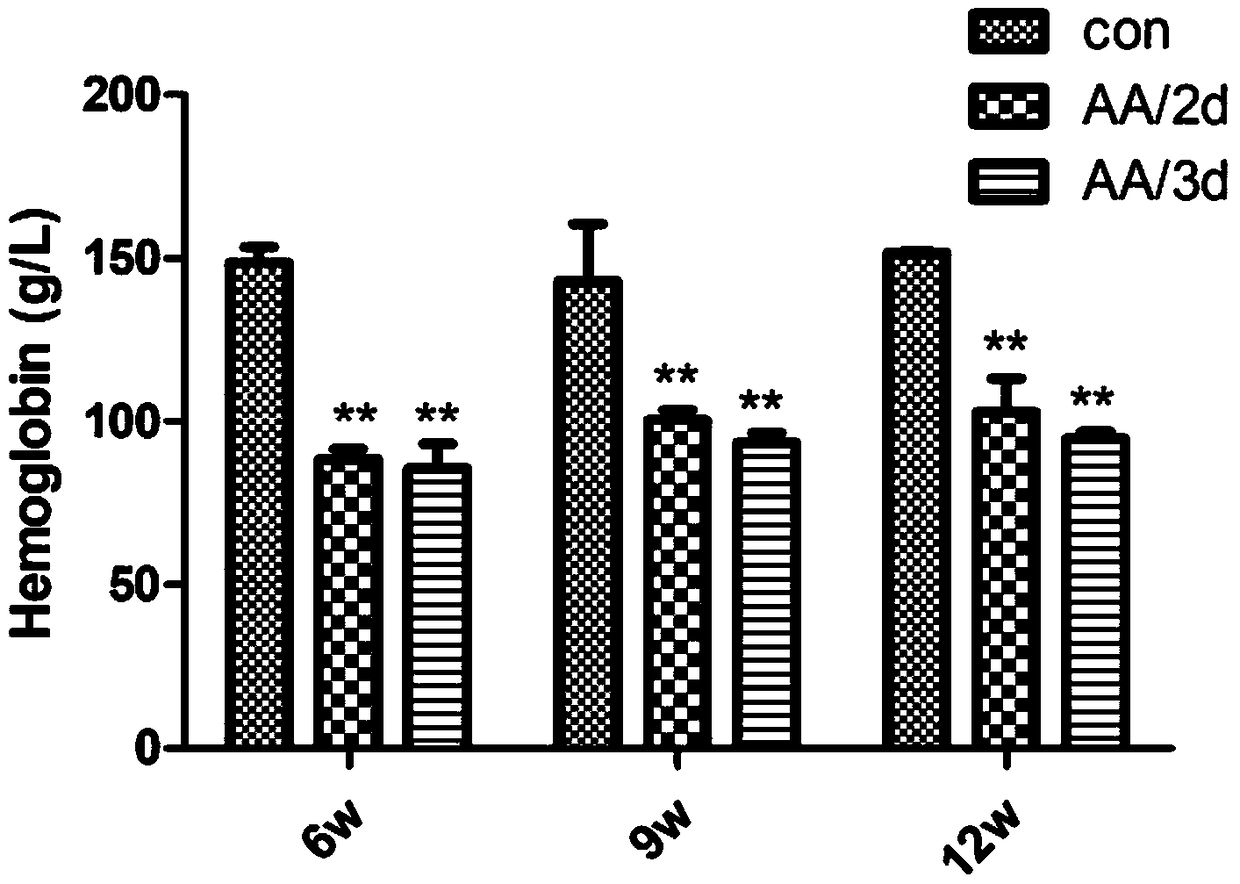
[0039] After 6 weeks of injection, the administration was stopped, and the renal function (serum creatinine, blood urea nitrogen), anemia (hemoglobin, hematocrit), and erythropoietin (EPO) levels of the three groups were detected, and the pathological changes of the kidneys were observed to determine the occurrence of Renal impairment and anemia.
[0040] 1. Renal function (serum creatinine, blood urea nitrogen) detection
[0041] The mice were injected intraperitoneally with aristolochic acid for 6 weeks, and continued to observe until 9 weeks and 12 weeks after drug withdrawal, and the above three time point indicators of serum creatinine (Scr) and blood urea nitrogen (BUN) were detected by enzymatic method. Serum creatinine indicators such as Figure 1A As shown, the serum creatinine level of the aristolochic acid AA / 2d group and AA / 3d group increased significantly at 6 weeks, compared with the control group, the difference...
Embodiment 3
[0129] Embodiment 3 model correction test
[0130] In order to confirm that the model of aristolochic acid-induced renal injury is a renal anemia model, the inventors gave EPO (3.5IU / g subcutaneous 1 / week) to the model group (AA / 3d group) after stopping the administration of aristolochic acid. At 6 weeks, the hemoglobin levels of the three groups (control group, model group, and model group treated with EPO) were measured. It was found that erythropoietin can significantly correct the renal anemia induced by aristolochic acid, and the hemoglobin in the model group rose from 94g / L to 141g / L, and basically returned to normal. This anemia model was verified again as a renal anemia model, and the results are shown in Table 1 Show.
[0131] Table 1 Comparison of hemoglobin in each group after EPO
[0132]
[0133] Note: Compared with control mice, *** P▲▲▲ P<0.001
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com