Recombinant protein prepared based on SIRP-alpha D1 mutant and application
A recombinant protein and mutant technology, applied in the field of genetic engineering, can solve the problems of not improving the phagocytosis of macrophages to cancer cells, not improving the targeting affinity of cancer cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
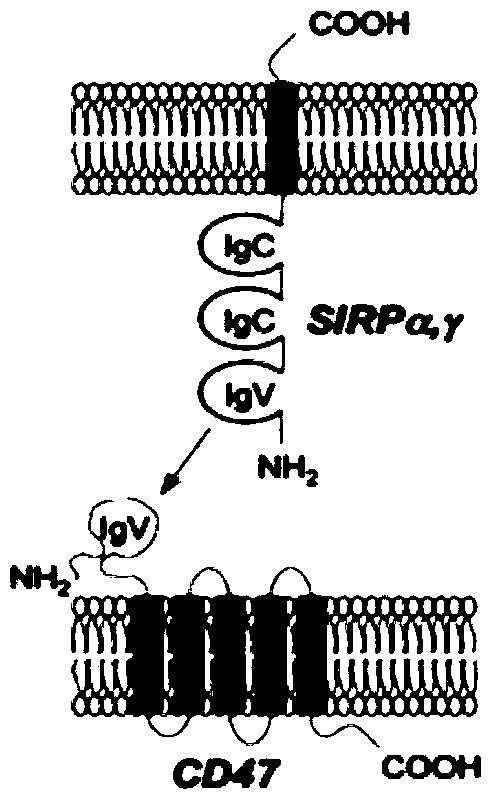
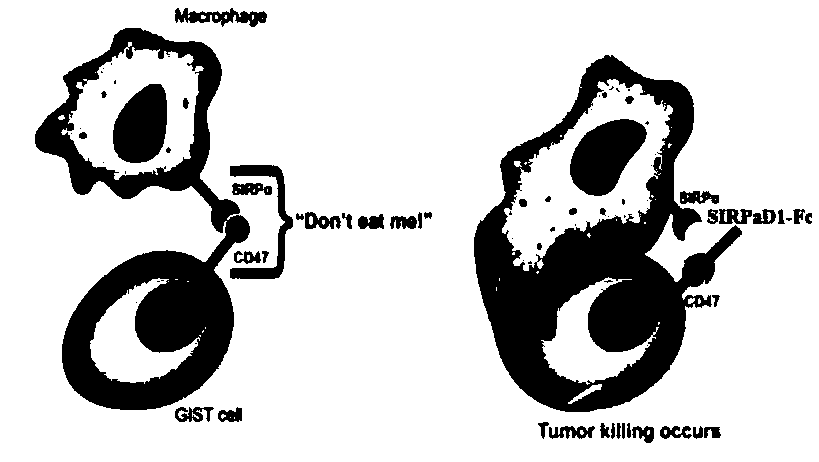
[0054] The present invention relates to two recombinant proteins, including SIRPαD1 contact surface amino acid point mutation combination coupled with human IgG1-Fc (CD001), and deglycosylation point mutation (N80A) on CD001 to obtain CD002. The above recombinant protein can be obtained through two mechanisms To kill tumor cells, one is to block the SIRPα-CD47 signal to activate the phagocytosis of tumor cells by macrophages, and the other is to combine Fc with the Fc receptor of macrophages to activate the immune function of macrophages. The present invention uses human embryonic kidney cells (293F) to express and purify the recombinant protein, and finds that the mutant has better target binding activity and macrophage ADCP activation function, and has a lower degree of hemolysis to red blood cells.
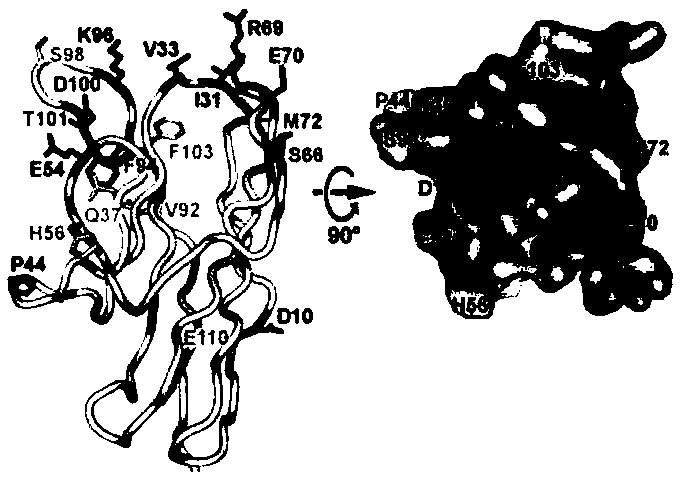
[0055]In the present invention, CD001 (I31 / K53 / E54 / H56 / S66) is obtained by point mutation of part of the surface amino acids in the binding region of SIRPαD1 and CD47, or the 80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com