Process for extracellular secretion of brazzein
A technology of sweet protein and plant, applied in the field of extracellular secretion of plant sweet protein, which can solve the problems of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
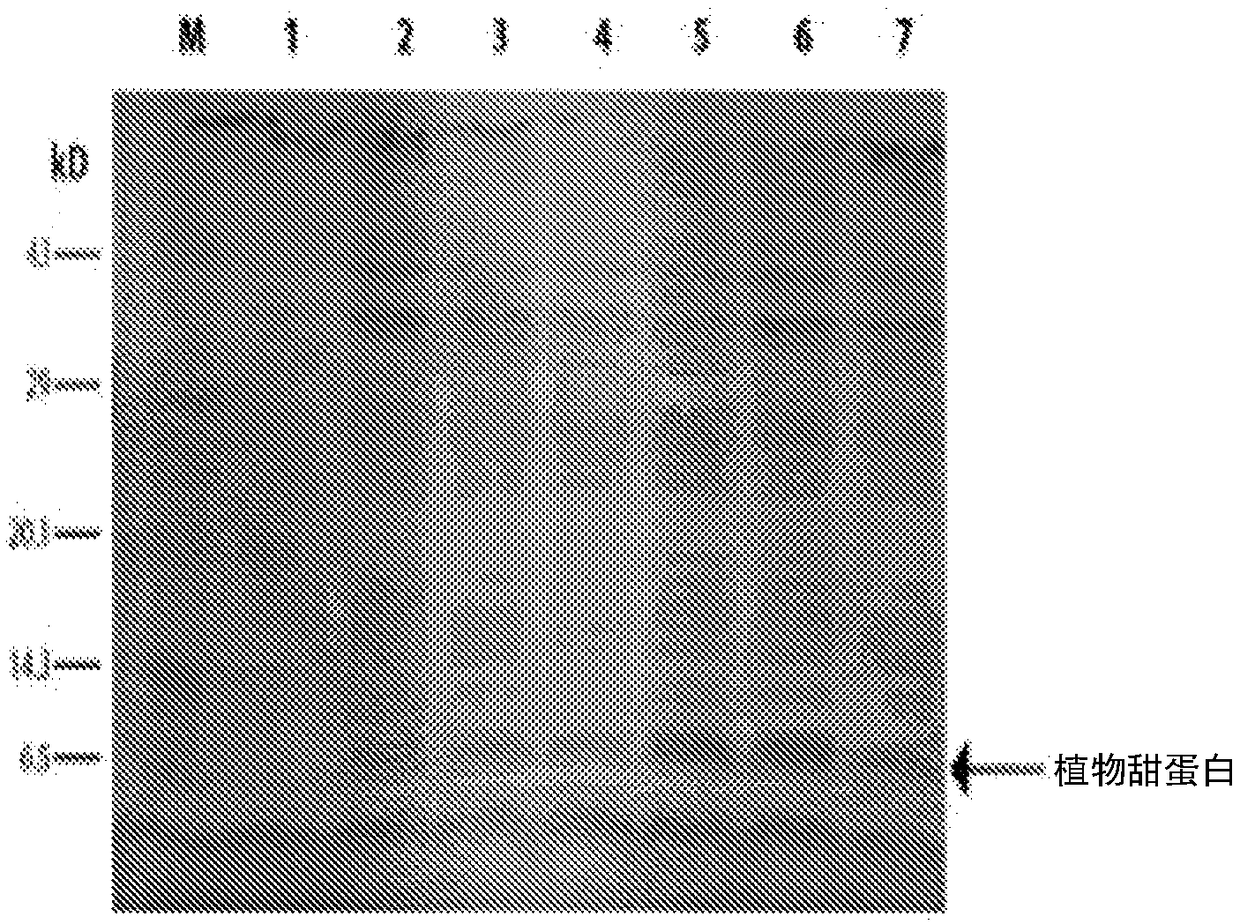
Examples
Embodiment 1
[0064] Example 1: Codon optimization and gene synthesis:
[0065] The amino acid sequence of the type III form of the plant sweet protein was retrieved from accession P56552 (Ming D et al., FEBS Lett. 355: 106-108 (1994)). The amino acid sequence was reverse-translated into a codon-optimized nucleotide sequence for E. coli. The codon-optimized gene also included an aspartic acid 28 to alanine mutation. This variant was previously shown to exhibit a better sweet taste profile than wild-type type III plant sweetin (Assadi-Porter FM et al., JL.; Arch Biochem Biophys. 2000 Apr 15;376(2):259-65).
[0066] The codon-optimized gene was fused at the N-terminus to a sequence encoding the pelB leader sequence and at the C-terminus to three tandem stop codons.
[0067] The final codon-optimized nucleotide sequence of pelB-phytosweetin is shown in SeqID 1 and the corresponding amino acid translation is shown in SeqID 2.
[0068] The pelB-phytosweetin gene was synthesized by Genscript (...
Embodiment 2
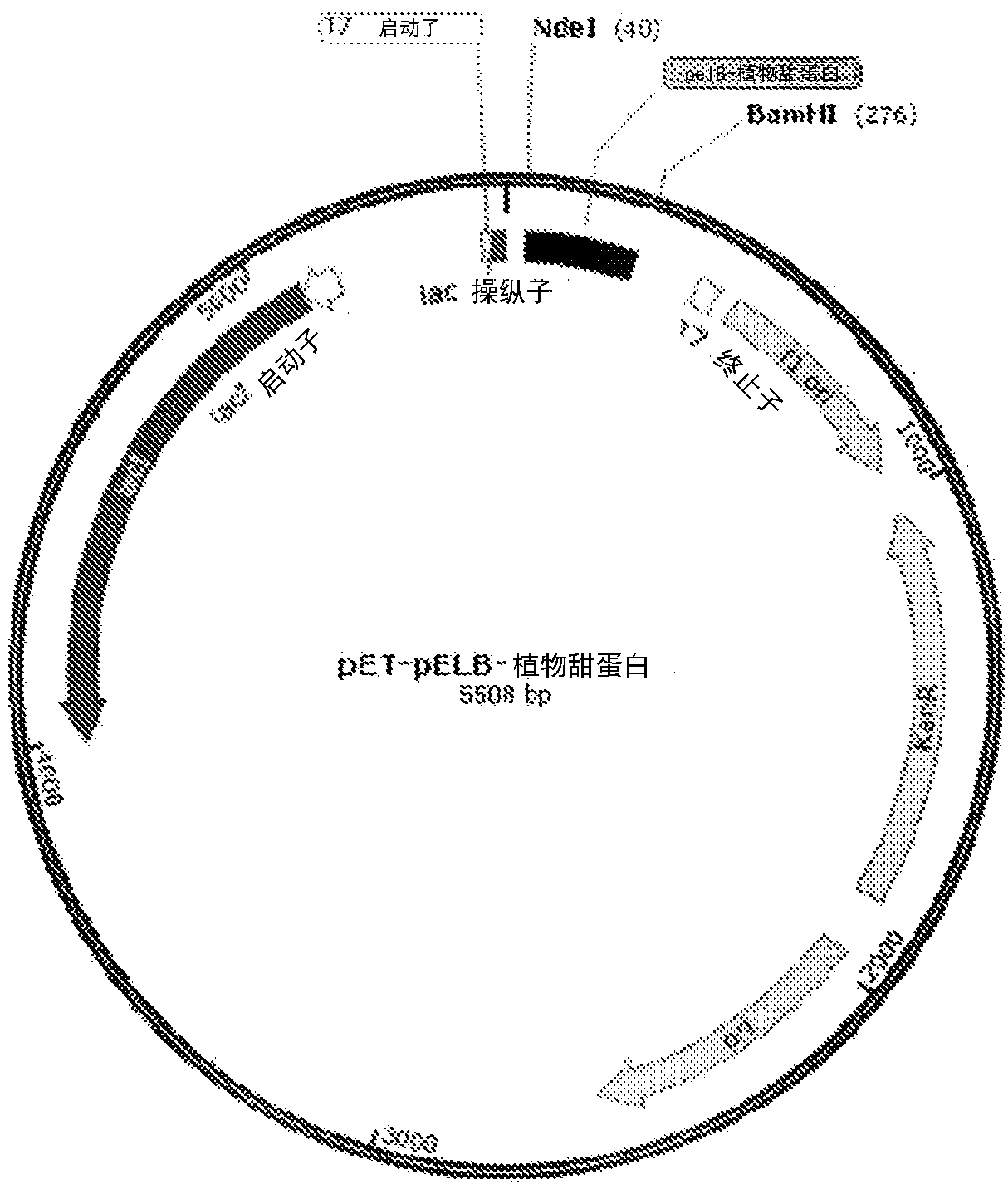
[0069] Embodiment 2: Construction of pET-pelB-plant sweet protein
[0070] The PCR reaction setup is provided in Table 1 below:
[0071] Table 1
[0072]
[0073]
[0074] Table 2
[0075]
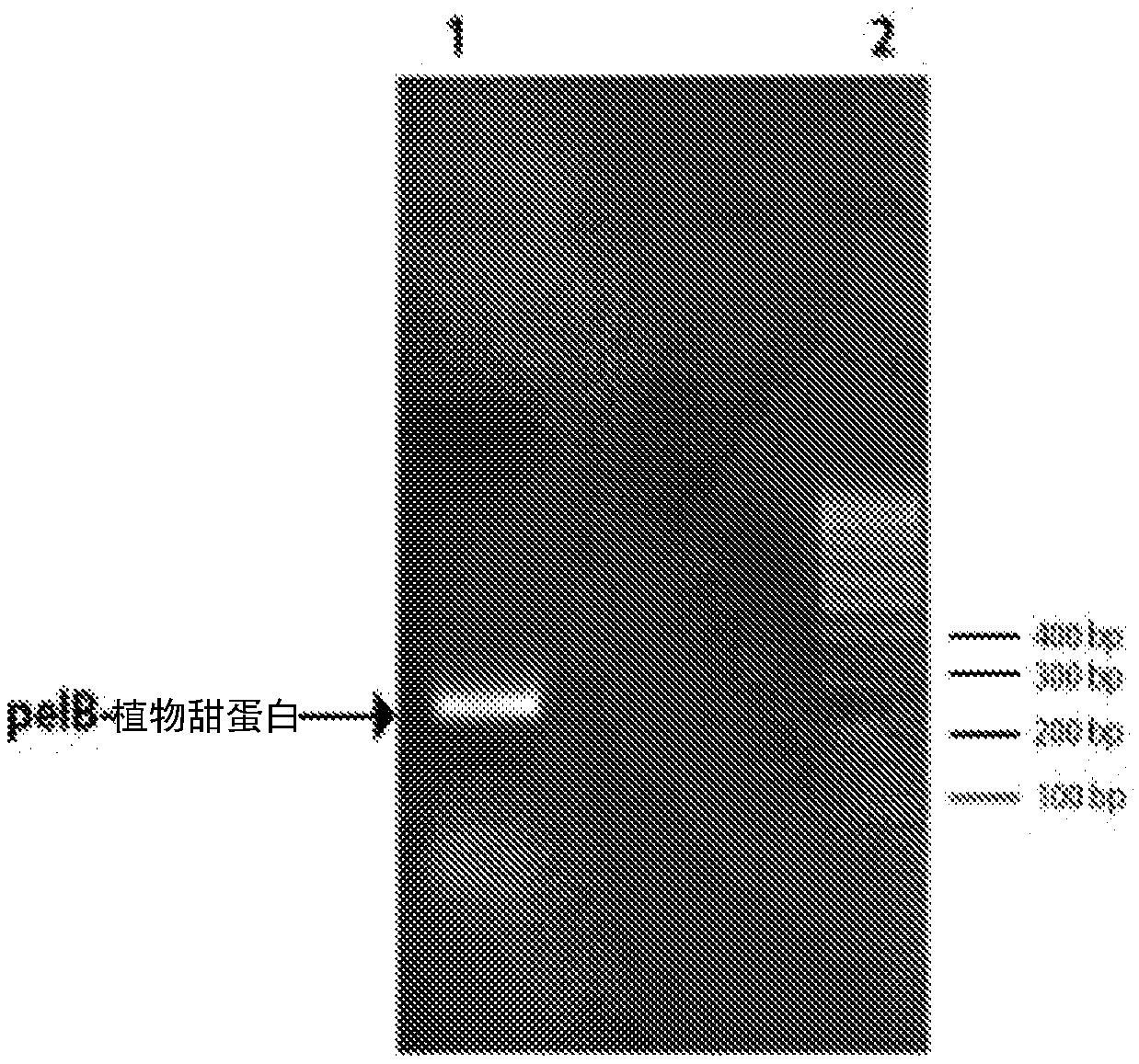
[0076] PCR amplification reactions were analyzed on a 1.6% (w / v) agarose gel. Excise about 250bp PCR amplification products corresponding to pelB-plant sweet protein ( figure 2 ), and purified using commercially available kits. The purified product was digested with NdeI and BamHI at 37°C for 4 hours and purified using a PCR spin column kit. It was ligated with pET-28a vector previously digested with NdeI and BamHI and gel purified. The ligation mix was transformed into DH5α competent cells. Transformed cells were plated on LB plates containing 50 μg / mL kanamycin and incubated overnight at 37°C. A single colony was picked from the plate into 5 mL LB broth containing 50 ug / mL kanamycin and grown in an orbital shaker at 37°C and 210 rpm for 16 hours. Plasmid DNA was isolate...
Embodiment 3
[0077] Embodiment 3: Construction of pET-pelB-plant sweet protein (A28D)
[0078] To generate wild type III phytosweetin, amino acid residue 50 of pelB-phytosweetin was mutated from alanine to aspartic acid and named pelB-phytosweetin (A28D).
[0079] The first PCR reaction was set up as follows: final PELBRAZ plasmid, primer PelBLun-FNcoNde (Seq IdNo.3), primer BRAZ-A28DRSOE (Seq Id No.5), Pfu-X reaction buffer, dNTP mix, Pfu-X polymerase And sterile water was used to make the final PCR reaction solution volume to 50 μL. Specific concentrations of the components are provided in Table 3.
[0080] Seq Id No.3:
[0081] 5'-GCGCGCCCATGGCATATGAAATACCTGCTGCCGACCGC-3'
[0082] Seq Id No.5:
[0083] 5'-CACCGCTACGCGCATGTTTTATCCAGTTTACAGTCGTAGTTACATTG GTTCGC-3'
[0084] Table 3: PCR solutions for the first reaction
[0085] components
concentration
Final PELBRAZ plasmid
50ng
PelBLun-FNcoNde (Seq Id No.3)
10 pmol
Braz-A28DRSOE (Seq Id No.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com