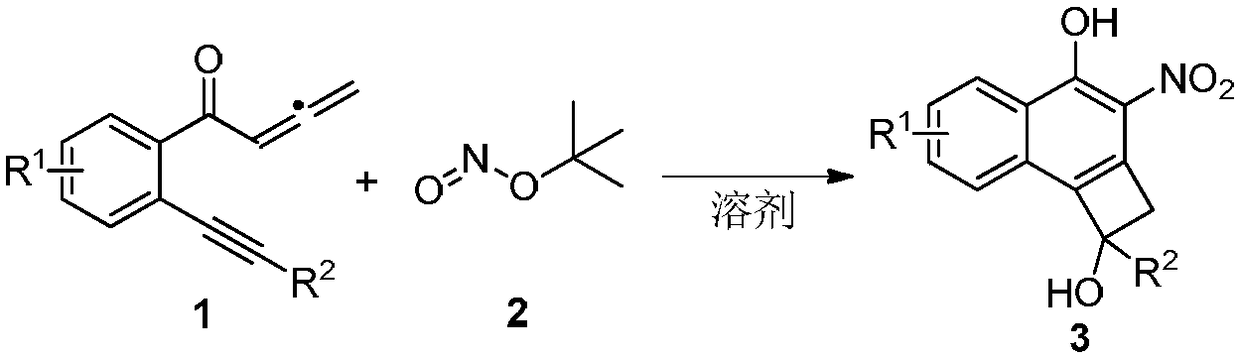
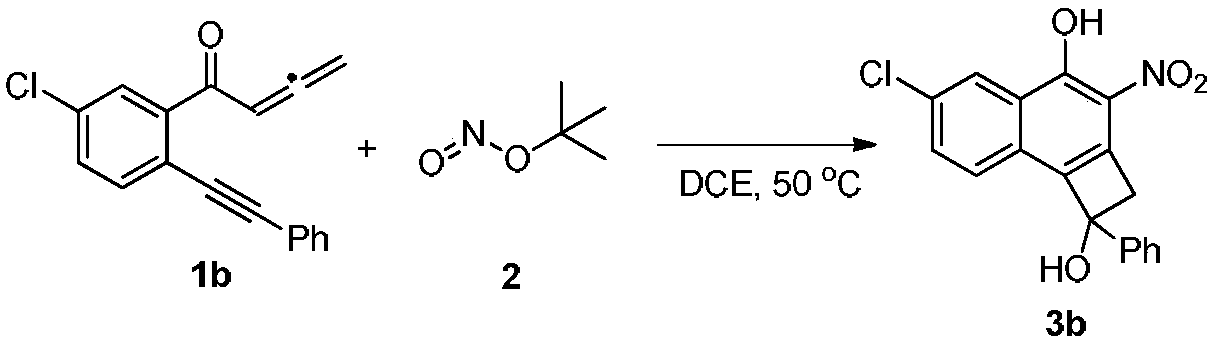
Synthesis method of cyclobutanol nitro-substituted naphthol compound
A synthesis method and compound technology, applied in the preparation of nitro compounds, organic chemistry, etc., can solve the problems of a large number of by-products, low product yield, expensive reagents, etc., and achieve simple operation, high atom economy, and wide application range. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
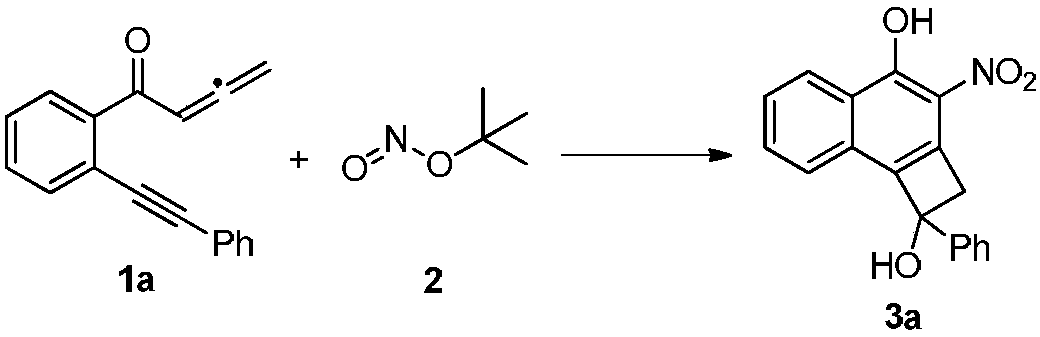
[0015] 1a (0.5mmol, 122mg), 2 (1.5mmol, 178μL) and acetonitrile (3mL) were sequentially added into a reaction tube, and the reaction was stirred at 50°C for 3h under an air atmosphere. Then the solvent was spin-dried and separated by silica gel column (petroleum ether / ethyl acetate=10 / 1) to obtain orange solid product 3a (77 mg, 50%). The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ:3.03(m,1H),3.80(d,J=14.8Hz,1H),3.86(d,J=14.4Hz,1H),7.28-7.34(m,3H),7.42-7.45(m,2H ),7.54-7.58(m,1H),7.61-7.68(m,2H),8.54(d,J=8.4Hz,1H),12.13(s,1H). 13 C NMR (100MHz, CDCl 3 )δ: 51.5, 81.1, 122.9, 125.2, 125.3, 125.9, 126.4, 126.7, 127.8, 128.6, 132.0, 132.2, 134.0, 136.2, 142.3, 156.9. HRMS calcd for C 18 h 13 NO 4 :306.0772[M-H] - ,found: 306.0775.
Embodiment 2
[0017] 1a (0.5mmol, 122mg), 2 (1.5mmol, 178μL) and toluene (3mL) were sequentially added into the reaction tube, and the reaction was stirred at 50°C for 3h under air atmosphere. Then the solvent was spin-dried and separated by silica gel column (petroleum ether / ethyl acetate=10 / 1) to obtain the orange solid product 3a (49 mg, 32%).
Embodiment 3
[0019] 1a (0.5mmol, 122mg), 2 (1.5mmol, 178μL) and 1,4-dioxane (3mL) were sequentially added into the reaction tube, and the reaction was stirred at 50°C for 3h under air atmosphere. Then the solvent was spin-dried and separated by silica gel column (petroleum ether / ethyl acetate=10 / 1) to obtain the orange solid product 3a (62 mg, 40%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com