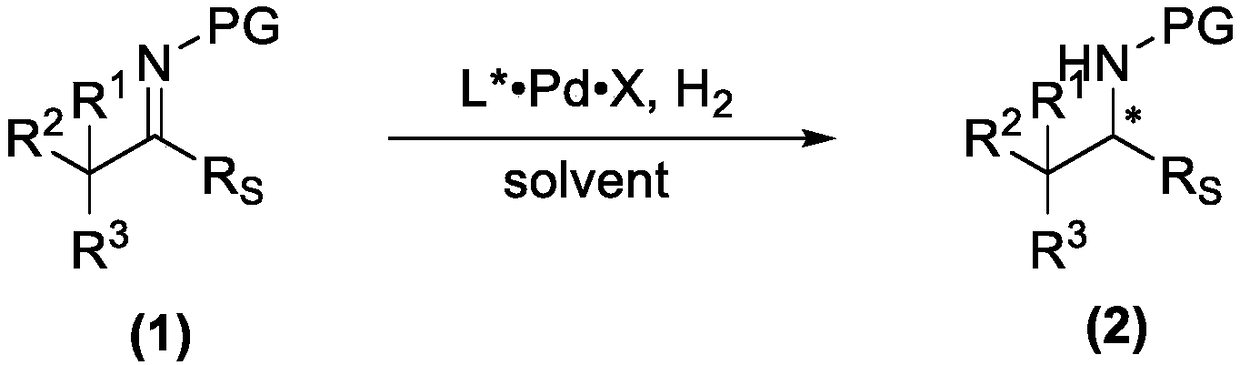Method for preparing high-steric-hindrance chiral amines from Z-type high-steric-hindrance imines through asymmetric palladium-catalyzed hydrogenation
A hindered imine, asymmetric technology, applied in the field of asymmetric palladium-catalyzed hydrogenation of Z-type hindered imines to prepare hindered chiral amines, achieving good reaction efficiency, mild conditions, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 2aa(R 1 = R 2 = R 3 =CH 3 , R S =C 6 h 5 , PG=Ts p-toluenesulfonyl) preparation
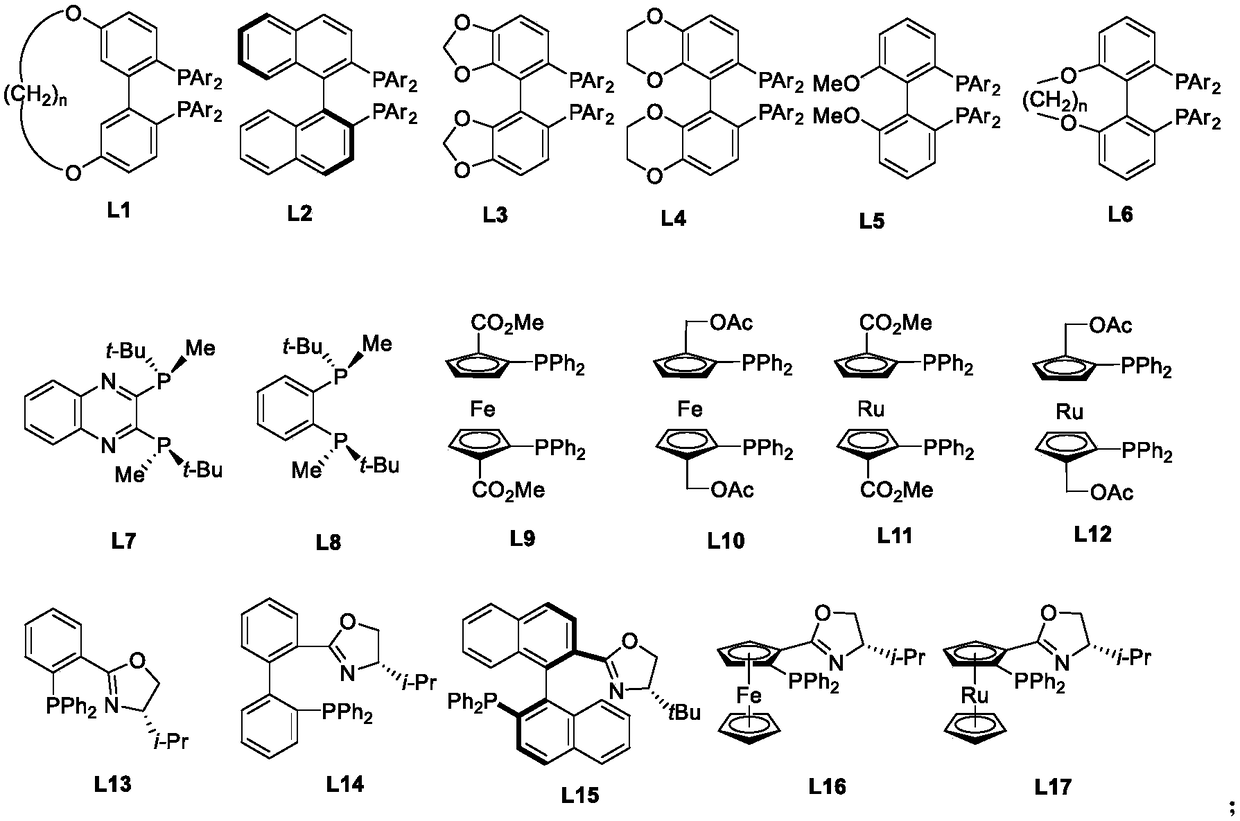
[0048] In a 10mL Schlenck tube, add phosphine ligand L1a (0.006mmol) and palladium trifluoroacetate (1.7mg, 0.005mmol), the system passed through the vacuum line, replaced with nitrogen for 3 times, added freshly distilled degassed acetone, the solution was in Stir at room temperature for 1 hour, and remove the solvent under reduced pressure to obtain a brown solid. After evacuating for 2 hours, add 2 mL of trifluoroethanol solvent, and add this solvent into a vial containing Z-type bulky hindered imine 1aa (0.25 mmol). Put it into an autoclave, and after 6 hydrogen replacements, the initial hydrogen pressure was 1 bar, and the reaction was stirred at room temperature for 24 hours. Cool, release gas carefully, open the autoclave, take out the vial, drain the solvent, detect the conversion by NMR, and obtain the product by column chromatography. Yield 93%, enantiomeric excess 95%. ...
Embodiment 2
[0050] 2aa(R 1 = R 2 = R 3 =CH 3 , R S =C 6 h 5 , PG=Ts) preparation
[0051] In a 10mL Schlenck tube, add phosphine ligand L1b (0.006mmol) and palladium trifluoroacetate (1.7mg, 0.005mmol), the system passed through the vacuum line, replaced with nitrogen for 3 times, added freshly distilled degassed acetone, the solution was in Stir at room temperature for 1 hour, and remove the solvent under reduced pressure to obtain a brown solid. After evacuating for 2 hours, add 2 mL of trifluoroethanol solvent, and add this solvent into a vial containing Z-type bulky hindered imine 1aa (0.25 mmol). Put it into an autoclave, and after 6 hydrogen replacements, the initial hydrogen pressure was 1 bar, and the reaction was stirred at room temperature for 24 hours. Cool, release gas carefully, open the autoclave, take out the vial, drain the solvent, detect the conversion by NMR, and obtain the product by column chromatography. Yield 92%, enantiomeric excess 98%. 2aa: white solid, ...
Embodiment 3
[0053] 2aa(R 1 = R 2 = R 3 =CH 3 , R S =C 6 h 5 , PG=Ts) preparation
[0054] In a 10mL Schlenck tube, add phosphine ligand L1c (0.006mmol) and palladium trifluoroacetate (1.7mg, 0.005mmol), the system passed through the vacuum line, replaced with nitrogen for 3 times, added freshly distilled degassed acetone, the solution was in Stir at room temperature for 1 hour, and remove the solvent under reduced pressure to obtain a brown solid. After evacuating for 2 hours, add 2 mL of trifluoroethanol solvent, and add this solvent into a vial containing Z-type bulky hindered imine 1aa (0.25 mmol). Put it into an autoclave, and after 6 hydrogen replacements, the initial hydrogen pressure was 1 bar, and the reaction was stirred at room temperature for 24 hours. Cool, release gas carefully, open the autoclave, take out the vial, drain the solvent, detect the conversion by NMR, and obtain the product by column chromatography. Yield 86%, enantiomeric excess 91%. 2aa: white solid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com