Preparation method and product of venetoclax
A technology of venetoclax and chlorophenyl, applied in the field of medicine, can solve the problems of increasing the production cost of enterprises, reducing the yield of the target product, reducing the activity of the amino group, etc., improving the quality, being beneficial to large-scale industrial production, and having fewer synthesis steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
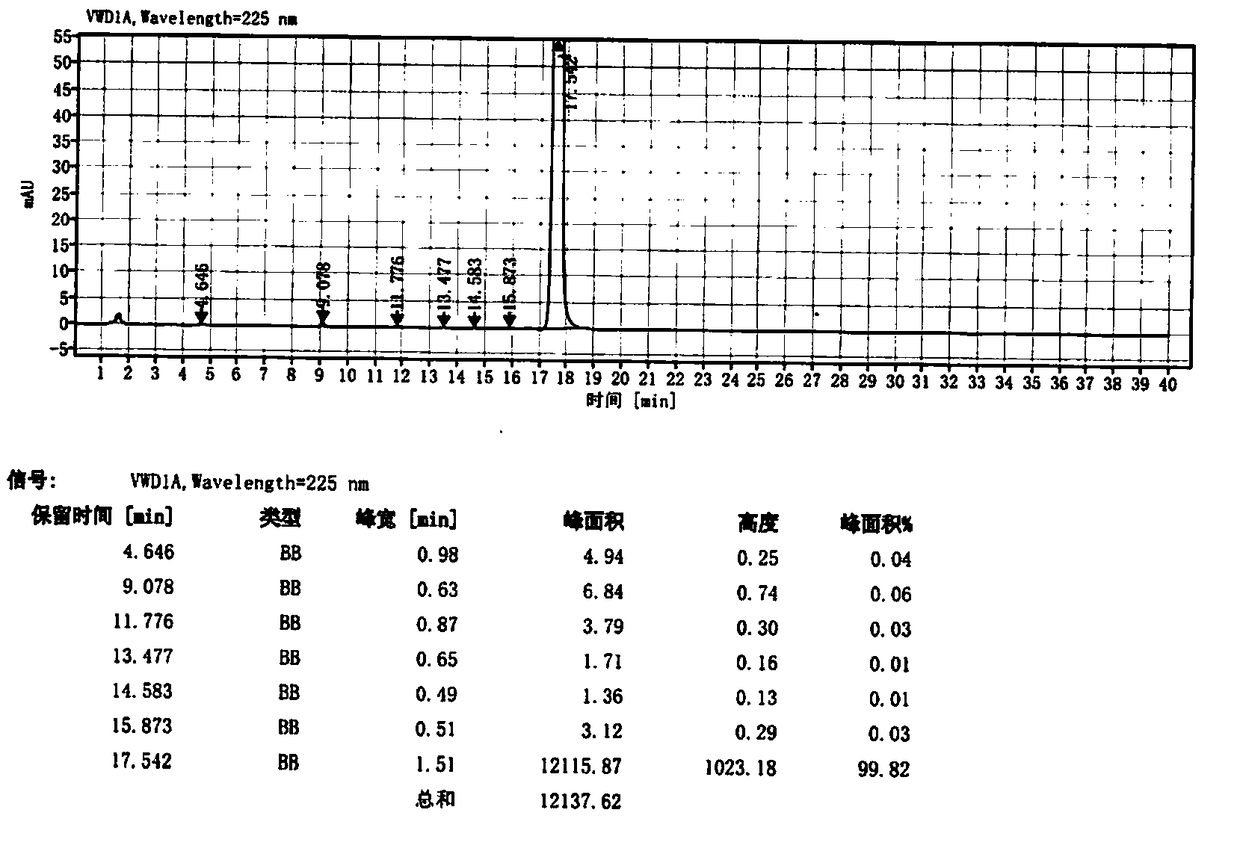
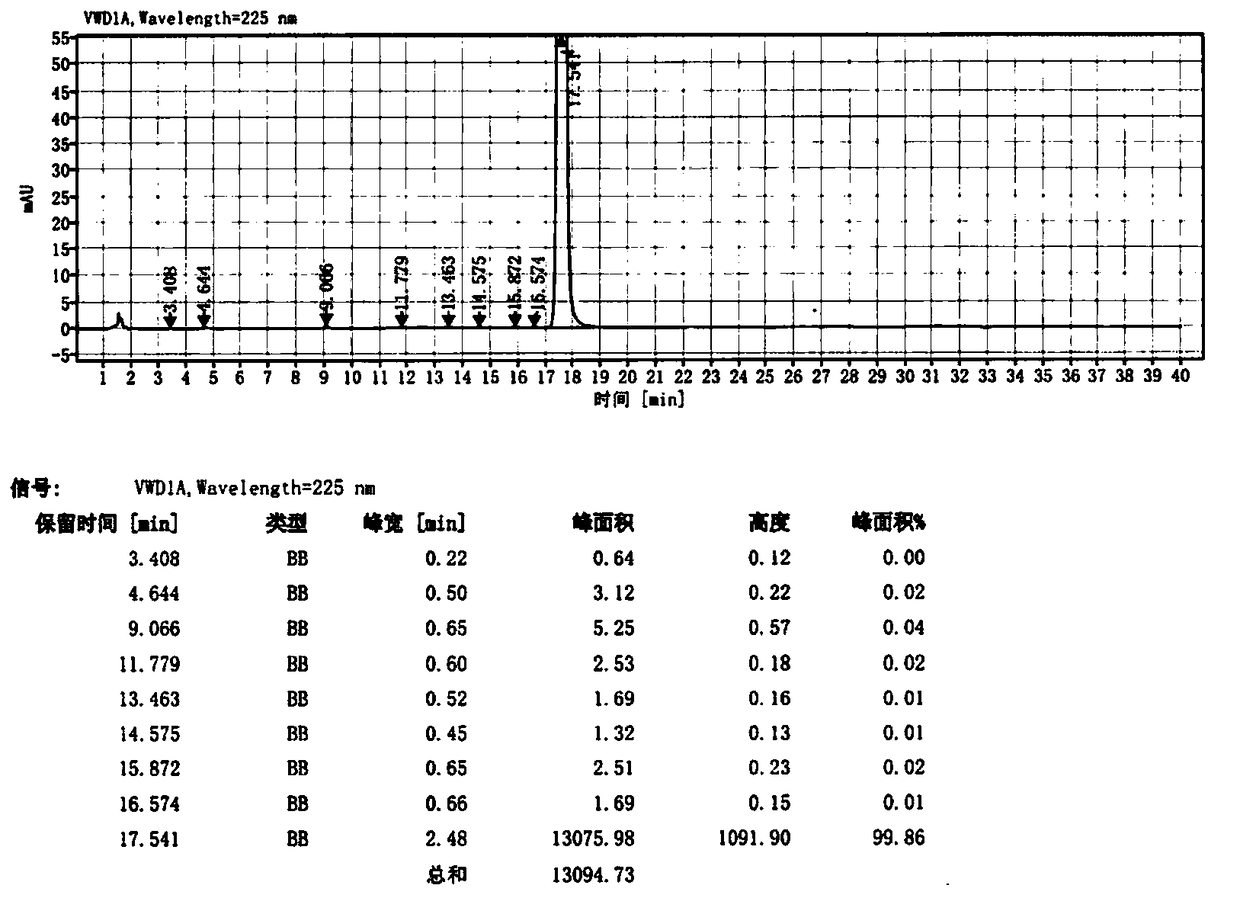
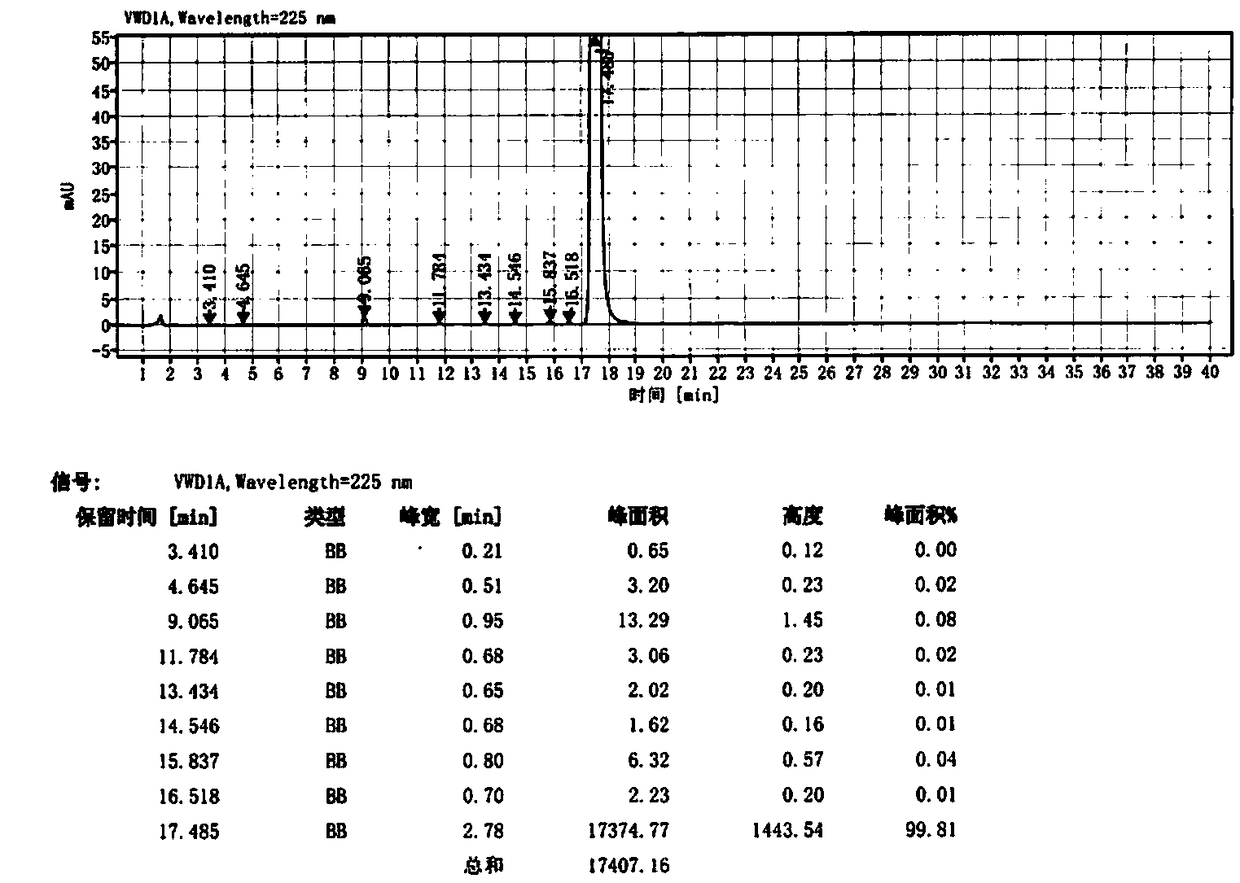
Image
Examples
Embodiment 1
[0036] Step 1: 4-(4-{[2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N -[(3-nitro-4-fluorophenyl)sulfonyl)]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide / 4-(4- {[2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-[(3-nitro- Preparation of 4-chlorophenyl)sulfonyl)]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide (Ⅲ)
[0037]
[0038] Compound (II) (20.0g, 35.0mmol) was dissolved in 300mL dichloromethane (DCM), and 3-nitro-4-fluorobenzenesulfonamide (8.5g, 38.5mmol), 4-di Methylaminopyridine (DMAP) (6.4 g, 52.5 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCL) (10.1 g, 52.5 mmol). Control the temperature at 20-30°C and stir the reaction for 16 hours. After completion of the reaction, add 100 mL of 5% acetic acid aqueous solution to wash once, and then wash once with 100 mL of water. The organic phase is dried with 20 g of sodium sulfate, filtered, and spin-dried to obtain 26.1 g of fluorine-containing c...
Embodiment 2
[0043] Step 1: 4-(4-{[2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N -[(3-nitro-4-fluorophenyl)sulfonyl)]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide / 4-(4- {[2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-[(3-nitro- Preparation of 4-chlorophenyl)sulfonyl)]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide (Ⅲ)
[0044]
[0045] Compound (II) (20.0g, 35.0mmol) was placed in 300mL of dichloromethane (DCM), and 3-nitro-4-chlorobenzenesulfonamide (9.1g, 38.5mmol), 4-di Methylaminopyridine (DMAP) (6.4 g, 52.5 mmol), 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCL) (10.1 g, 52.5 mmol). The temperature was controlled at 20-30° C. and stirred for 16 hours. TLC detection showed that the reaction of the raw materials was complete. After completion of the reaction, add 100 mL of 5% acetic acid aqueous solution to wash once, and then wash once with 100 mL of water. The organic phase is dried with 20 g of sodium ...
Embodiment 3
[0050] Step 1: 4-(4-{[2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N -[(3-nitro-4-fluorophenyl)sulfonyl)]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide / 4-(4- {[2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-[(3-nitro- Preparation of 4-chlorophenyl)sulfonyl)]-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide (Ⅲ)
[0051]
[0052] Compound (Ⅱ) (20.0g, 35.0mmol) was dissolved in 100mL N,N-dimethylformamide (DMF), and 3-nitro-4-fluorobenzenesulfonamide (8.5g, 38.5mmol) was added successively at room temperature ), 1-hydroxybenzotriazole (HOBt) (7.1 g, 52.5 mmol), N,N'-diisopropylcarbodiimide (DIC) (6.6 g, 52.5 mmol). Control the temperature at 10-20° C. and stir the reaction for 12 hours. TLC detection shows that the reaction of the raw materials is complete. After the reaction is completed, add 300 mL of dichloromethane to mix, then add 100 mL of 1% acetic acid aqueous solution to wash once, the organic phase is washed onc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com