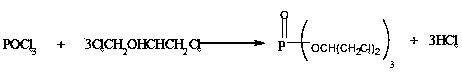
Method for preparing tris(1,3-dichloropropyl) phosphate
A technology of dichloropropyl and dichloropropanol is applied in the field of preparing phosphoric acid triester, which can solve the problems of complex preparation process, difficult catalyst handling and high cost, achieve high reactivity, improve raw material conversion rate, and mild process conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] At room temperature, add 153g of phosphorus oxychloride and 774g of 1,3-dichloropropanol (molar ratio: 1:6) into the reaction kettle, stir and raise the temperature to 115°C. After reacting at this temperature for 1 hour, under negative pressure Remove the hydrogen chloride gas generated by the reaction to the hydrogen chloride absorption device under the condition of -0.002MPa, and at the same time, the temperature is gradually increased to 180°C at a rate of 15°C / h, and the reaction is continued for 1 hour. The crude product after cooling is alkali washed, washed with water, and distilled to obtain triphosphate The (1,3-dichloropropyl) ester has a purity of 98.87% and a yield of 85.79%. The 1,3-dichloropropanol recovered by distillation is recycled as the reaction raw material.
Embodiment 2
[0023] At room temperature, 153g of phosphorus oxychloride and 903g of 1,3-dichloropropanol (molar ratio: 1:7) were added to the reaction kettle and stirred and heated to 110°C. After reacting at this temperature for 1.5 hours, it was heated under negative pressure. Remove the hydrogen chloride gas generated by the reaction to the hydrogen chloride absorption device under the condition of -0.003MPa, and at the same time, the temperature is gradually increased to 180°C at a rate of 20°C / h, and the reaction is continued for 2 hours. The crude product after cooling is alkali washed, washed with water, and distilled to obtain triphosphate The (1,3-dichloropropyl) ester has a purity of 99.01% and a yield of 87.42%. The 1,3-dichloropropanol recovered by distillation is recycled as the reaction raw material.
Embodiment 3
[0025] At room temperature, add 153 g of phosphorus oxychloride and 645 g of 1,3-dichloropropanol (molar ratio: 1:5) into the reaction kettle, stir and raise the temperature to 120°C. After reacting at this temperature for 1 hour, under negative pressure Remove the hydrogen chloride gas generated by the reaction to the hydrogen chloride absorption device under the condition of -0.003MPa, and at the same time, the temperature is gradually increased to 180°C at a rate of 30°C / h, and the reaction is continued for 2 hours. The crude product after cooling is alkali washed, washed with water, and distilled to obtain triphosphate The (1,3-dichloropropyl) ester has a purity of 98.77% and a yield of 86.83%. The 1,3-dichloropropanol recovered by distillation is recycled as the reaction raw material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com