Liver fibrosis marker control material and preparation method thereof
A technology for liver fibrosis and quality control materials, which is applied in the preparation of test samples, chemiluminescence/bioluminescence, and analysis by chemical reaction of materials, etc. Good uniformity and stability, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of Liver Fibrosis Marker Substance Control
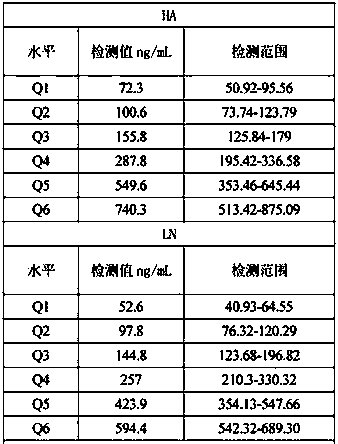
[0024] Six levels of liver fibrosis markers (Q1: HA-70ng / mL, LN-70ng / mL, PIIINP-5ng / mL, CIV-50ng / mL, CG-1.5μg / mL; Q2: HA-100ng / mL , LN-100ng / mL, PIIINP-10ng / mL, CIV-100ng / mL, CG-2.5μg / mL; Q3: HA-150ng / mL, LN-150ng / mL, PIIINP-15ng / mL, CIV-150ng / mL mL, CG-5.0μg / mL; Q4: HA-250ng / mL, LN-250ng / mL, PIIINP-35ng / mL, CIV-250ng / mL, CG-10μg / mL; Q5: HA-500ng / mL, LN -500ng / mL, PIIINP-50ng / mL, CIV-500ng / mL, CG-25μg / mL; Q6: HA-700ng / mL, LN-700ng / mL, PIIINP-70ng / mL, CIV-700ng / mL, CG -40 μg / mL).
[0025] 1. Select healthy adults who are negative for infectious markers such as HBsAg, anti-HCV, anti-specific TP, and anti-HIV in blood as qualified blood donors, and collect liver fibrosis-negative mixed serum;
[0026] 2. Treat the above-mentioned hepatic fibrosis-negative mixed serum in a 56°C water bath for 2 hours, then centrifuge it in a high-speed centrifuge (3500r / min≤speed≤8000r / min) for 10 minutes, and store it at...
Embodiment 2
[0032] Embodiment 2 The performance test of the quality control product of the present invention
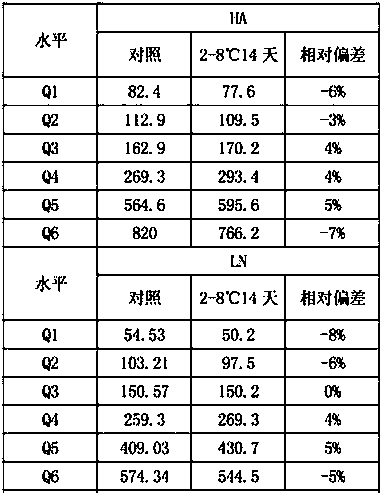
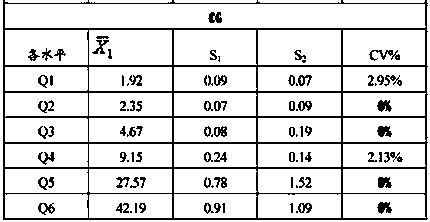
[0033] The homogeneity and stability of liver fibrosis marker controls were assessed. Through the statistical analysis of the performance evaluation results, it is verified whether the indicators meet the requirements of the experimental design.
[0034] 1. Uniformity verification
[0035] Take 10 bottles of each level of quality control products of the same batch number, each bottle of quality control products is tested once with the corresponding reagent, and the average value of the 10 test results is calculated according to formulas 1 and 2 ( ) and standard deviation S1;
[0036] In addition, use 1 bottle of the above-mentioned 10 bottles of quality control products for 10 consecutive tests, and calculate the average value of 10 test results ( ) and standard deviation S2; calculate the coefficient of variation CV between bottles according to the following formula, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com