Hemostatic composition, and preparation method and application of hemostatic composition
A composition and coagulation technology, applied in application, pharmaceutical formulation, drug delivery, etc., can solve the problems such as subsequent swelling properties of hemostatic composite materials that are not involved, and achieve the effect of avoiding auxiliary tools and increasing gel strength.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
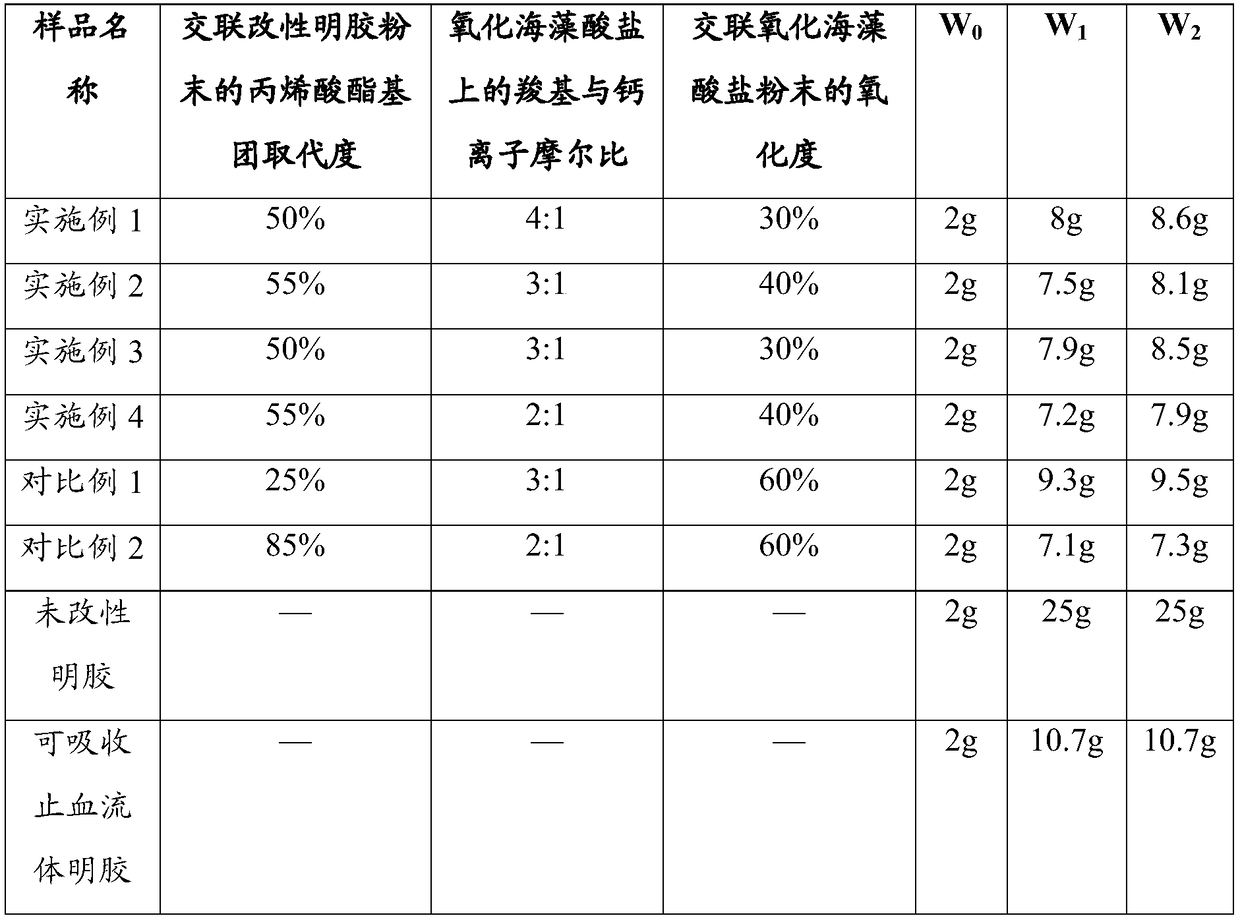
[0043] The preparation method of the hemostatic composition of the present invention comprises 1) preparation of cross-linked modified gelatin powder; 2) preparation of cross-linked oxidized alginate powder; 3) preparation of hemostatic composition. A detailed description is given below.
[0044] In step 1), 0.1 to 1 part by weight, preferably 0.5 to 0.8 parts by weight of (meth)acrylated gelatin and 0.005 to 0.1 parts by weight, preferably 0.01 to 0.05 parts by weight of β-cyclodextrin and The embedding formed by camphorquinone is added to 0.5-2 parts by weight, preferably 1.6-1.8 parts by weight of phosphate (PBS) buffer solution, and then the wavelength is 420nm-480nm, and the light intensity is 1200-2000mW / cm 2 Irradiate blue light for 3 to 5 minutes to solidify and cross-link to obtain a cross-linked product, remove residual embedded matter, freeze-dry at -80 to -35°C, preferably -60 to -40°C, crush and sieve to obtain granules Cross-linked modified gelatin powder having...
Embodiment 1
[0067] Preparation of methacrylated gelatin:
[0068] Add 10g of gelatin into 100ml of PBS buffer (0.01M), heat up to 50°C to dissolve completely, then slowly add 0.6ml of methacrylic anhydride dropwise, and keep warm for 3 hours after the dropwise addition to form a reaction solution. 400 ml of PBS buffer (0.01M) was added to the reaction solution. The diluted reaction solution was dialyzed with regenerated cellulose membrane ( Molecular weight cut-off 12000 ~ 14000) dialyzed for 5 days, and the purified water was replaced once a day. Using a 0.45 μm microporous filter membrane to filter under reduced pressure and freeze-dry to obtain methacrylic anhydride gelatin.
[0069] Visible photoinitiator:
[0070] Mix β-cyclodextrin, camphorquinone and water at a ratio of 2:1:10 (mass:mass:volume), and ultrasonically induce solubilization to form embeddings; then dry the embeddings under reduced pressure, grind them, and vacuum Dry to obtain a visible photoinitiator.
[0071] P...
Embodiment 2
[0079] Preparation of methacrylated gelatin:
[0080] Add 10g of gelatin into 100ml of PBS buffer (0.01M), raise the temperature to 50°C to dissolve completely, then slowly add 0.8ml of methacrylic anhydride dropwise, and keep warm for 3 hours after the dropwise addition to form a reaction solution. 400 ml of PBS buffer (0.01M) was added to the reaction solution. The diluted reaction solution was dialyzed with regenerated cellulose membrane ( Molecular weight cut-off 12000 ~ 14000) dialyzed for 5 days, and the purified water was replaced once a day. Using a 0.45 μm microporous filter membrane to filter under reduced pressure and freeze-dry to obtain methacrylic anhydride gelatin.
[0081] Visible photoinitiator:
[0082] Mix β-cyclodextrin, camphorquinone and water at a ratio of 2:1:10 (mass:mass:volume), and ultrasonically induce solubilization to form embeddings; then dry the embeddings under reduced pressure, grind them, and vacuum Dry to obtain a visible photoinitiato...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com