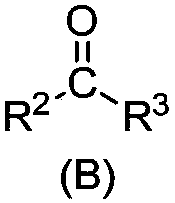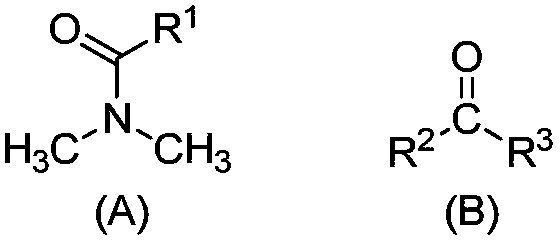Lithium extractant and method for extracting lithium from salt lake brine
A salt lake brine and extraction agent technology, applied in the field of extraction chemistry, can solve the problems of high polarity, low solubility and increase of TBP density molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
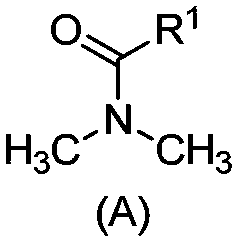
[0100] Treat the salt lake brine of the composition shown in Table 1 according to the general method, wherein the extracted organic phase is a mixture of N,N-dimethyloctylamide and D70 solvent oil, and the volume fractions are 40% and 60% respectively; as shown in Table 1 Add FeCl to brine 3 Mix and dissolve and make Fe / Li=1.5 (molar ratio) as the extraction feed liquid; extraction O / A=2.0; washing O / A=30; stripping agent: 6mol / LHCl, stripping O / A=30; regeneration Agent: 0.5mol / L NaOH solution, regeneration O / A=20.
[0101] After extraction, the system was divided into three clear and transparent phases. The single-stage lithium extraction rate is 61.5%, and the single-stage lithium-magnesium separation coefficient (β Li / Mg ), lithium-sodium separation coefficient (β Li / Na ), lithium-potassium separation coefficient (β Li / K ) reached 285.3, 21.5, 37.7 respectively. After 6 stages of extraction, 5 stages of washing, and 5 stages of stripping, the recovery rate of lithium r...
Embodiment 2
[0103] Treat the salt lake brine with the composition shown in Table 1 according to the general method, wherein the extracted organic phase is a mixture of N,N-dimethyloctylamide, diisobutyl ketone, and D70 solvent oil, and the volume fractions are 40%, 20%, and 40%; add FeCl to the brine shown in Table 1 3 Mix and dissolve and make Fe / Li=1.5 (molar ratio) as the extraction feed liquid; extraction O / A=2.0; washing O / A=20; stripping agent: 6mol / L HCl, stripping O / A=30; Regenerant: 0.5mol / L NaOH solution, regeneration O / A=20.
[0104] After extraction, the system is divided into two clear and transparent phases without an intermediate phase (the third phase). The single-stage lithium extraction rate is 68.7%, and the single-stage lithium-magnesium separation coefficient (β Li / Mg ), lithium-sodium separation coefficient (β Li / Na ), lithium-potassium separation coefficient (β Li / K ) reached 310.8, 30.4, 46.5 respectively. After 5 stages of extraction, 4 stages of washing, and...
Embodiment 3
[0106]The salt lake brine of the composition shown in Table 1 is processed according to the general method, wherein the extracted organic phase is a mixture of N,N-dimethyloctylamide, diisobutyl ketone, and 260# aviation kerosene, and the volume fractions are 30%, 30% respectively. %, 40%; add FeCl to the brine shown in Table 1 3 Mix and dissolve and make Fe / Li=1.3 (molar ratio) as the extraction feed liquid; extraction O / A=1.0; washing O / A=30; stripping agent: 6mol / L HCl, stripping O / A=30; Regenerant: 0.3mol / L Na 2 CO 3 solution, regeneration O / A=20.
[0107] After extraction, the system was divided into two phases. The single-stage lithium extraction rate is 55.8%, and the single-stage lithium-magnesium separation coefficient (β Li / Mg ), lithium-sodium separation coefficient (β Li / Na ), lithium-potassium separation coefficient (β Li / K ) reached 280.2, 20.3, 40.5 respectively. After 7 stages of extraction, 5 stages of washing, and 5 stages of stripping, the recovery ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com