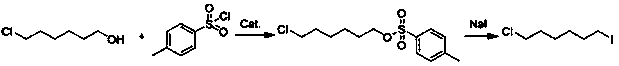Preparation method of 1-chloro-6-iodohexane
A technology of iodohexane and chlorohexyl ester, which is applied in the field of preparation of 1-chloro-6-iodohexane, can solve the problems of difficult operation and high cost, achieve easy control of the production process, reduce the difficulty of reaction, and reduce the difficulty of operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Synthesis of 4-methylbenzenesulfonic acid-6-chlorohexyl ester
[0016] Add 50mL sodium hydroxide aqueous solution (the sodium hydroxide is 4mol / L), 0.5gTEBA and 10.0g 6-chloro-1-hexanol into the reaction kettle, cool in ice / water bath, and slowly add 15.0g p-toluene dropwise under rapid stirring 50mL benzene solution of sulfonyl chloride, the reaction temperature is controlled at -5~0°C, the dropwise addition is completed in 2 hours, and the reaction is continued for 2 hours, left to stand, separated, dried, and benzene is recovered by atmospheric distillation to obtain 21.3g of 4-methylbenzenesulfonate Acid-6-chlorohexyl crude product (content 90%).
[0017] Synthesis of 1-chloro-6-iodohexane
[0018] Add the above crude product and 10.88g of sodium iodide into 50mL of acetone, stir vigorously, reflux for 1 hour, cool, stand, filter, and the filtrate is distilled under normal pressure to recover acetone, then rectified, and the fraction at 82°C is collected as product...
Embodiment 2
[0020] Synthesis of 4-methylbenzenesulfonic acid-6-chlorohexyl ester
[0021] Add 50mL sodium hydroxide aqueous solution (the sodium hydroxide is 4mol / L), 0.6gTEBA and 10.0g 6-chloro-1-hexanol into the reaction kettle, cool in ice / water bath, and slowly add 16.5g p-toluene dropwise under rapid stirring 50mL toluene solution of sulfonyl chloride, the reaction temperature is controlled at 0~5°C, the dropwise addition is completed in 2 hours, and the reaction is continued for 2 hours, left to stand, separated, dried, and the toluene is recovered by atmospheric distillation to obtain 21.6g of 4-methylbenzenesulfonic acid -Crude 6-chlorohexyl ester (content 91%).
[0022] Synthesis of 1-chloro-6-iodohexane
[0023] Add the above crude product and 11.97g of sodium iodide to 50mL of acetone, stir vigorously, reflux for 1 hour, cool, let stand, filter, the filtrate is distilled under normal pressure to recover acetone, and then rectified, and the fraction at 82°C is collected as prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com