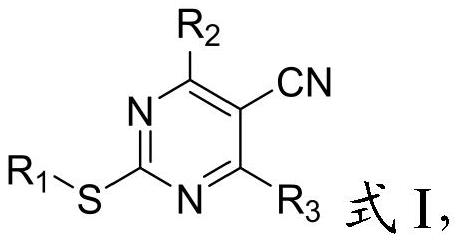
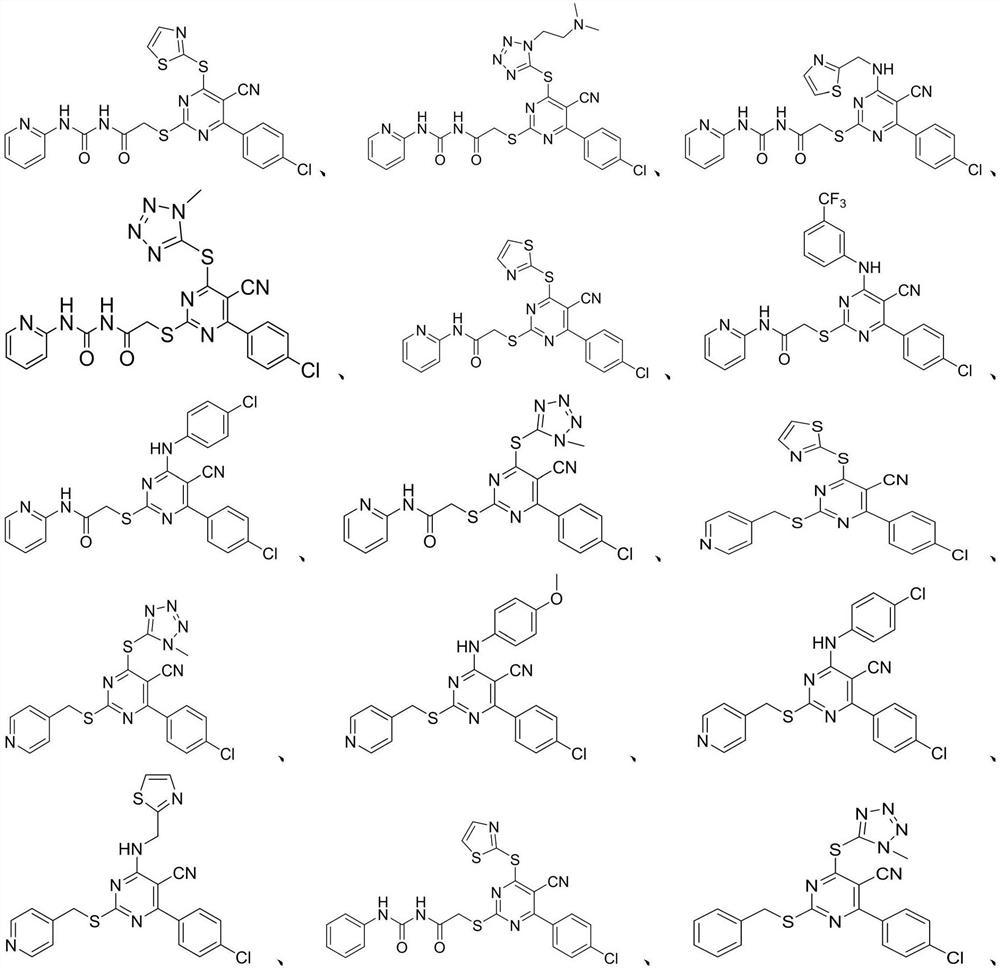
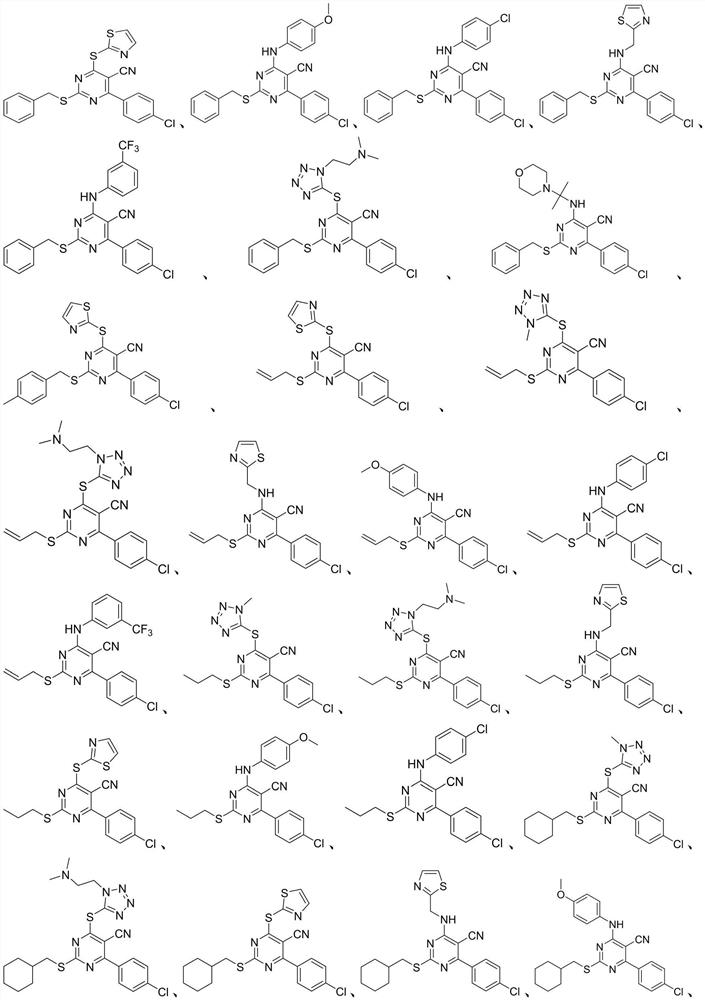
A kind of 2-mercapto-5-cyanopyrimidine derivatives and its preparation method and application
A technology of derivatives and mercapto groups, which is applied in the field of 2-mercapto-5-cyanopyrimidine derivatives and their preparation, can solve the problems of low bioavailability, poor selectivity, easy to produce drug resistance, etc., and achieve good The effect of inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention provides a preparation method of 2-mercapto-5-cyanopyrimidine derivatives described in the above technical scheme, comprising the following steps:
[0043] Ethyl cyanoacetate, thiourea, R 3 -CHO, alkali reagent and solvent I are mixed, and the ring closure reaction is carried out to obtain the compound having the structure shown in formula II;
[0044] The compound having the structure shown in formula II, R 1 -R 4 Mix with solvent II, carry out substitution reaction I, obtain the compound with the structure shown in formula III; Said R 1 -R 4 Middle R 4 Including -Br or -Cl;
[0045] Mixing the compound having the structure shown in formula III, phosphorus oxychloride and solvent III, and performing substitution reaction II to obtain the compound having the structure shown in formula IV;
[0046] The compound having the structure shown in formula IV, R 2 -H is mixed with solvent IV to carry out substitution reaction III to obtain 2-mercapto...
Embodiment 1
[0073] Add ethyl cyanoacetate (20mmol) and potassium hydroxide (30mmol) into ethanol (AR, 50mL), stir and dissolve at 90°C, then add thiourea (30mmol) and 3,4,5-trimethoxy Benzaldehyde (30 mmol) was added to the reaction system, and the stirring was continued at 90°C for 10 h (TLC tracking and detection of the reaction progress); the obtained system was subjected to suction filtration and recrystallization to obtain a compound having the structure shown in formula II-1;
[0074] The compound (20mmol) having the structure shown in formula II-1 was mixed with acetonitrile (AR, 50mL), m-trifluoromethylbenzyl bromide (30mmol) was added dropwise to the resulting solution, and stirred at 60°C for 6h (TLC tracking detection reaction process); the acetonitrile in the gained system is evaporated, and the mixture of ethyl acetate and water (the volume ratio of ethyl acetate and water is 1:1) is used to extract the residue, and the gained organic phase is subjected to chromatographic colu...
Embodiment 2
[0081] According to the method in Example 1, the compound of the structure shown in formula I-2 is prepared, the only difference is that m-trifluoromethyl benzyl bromide is replaced by 4-methyl benzyl bromide, and 1-(2-dimethylamino Ethyl)-1H-5-mercaptotetrazole is replaced by 2-mercaptothiazole;
[0082] The compound with the structure shown in formula I-2 is a white solid with a melting point of 106-107°C and a yield of 56%;
[0083]
[0084] The analysis results are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.98(d, J=3.0Hz, 1H, Ar-H), 7.62(d, J=3.1Hz, 1H, Ar-H), 7.31(s, 2H, Ar-H, 7.10(s, 4H ,Ar-H),4.08(s,2H,-CH 2 -),3.93(d,J=5.6Hz,9H,-CH 3 ),2.32(s,3H,-CH 3 ).HR-MS(ESI),calcd.C 25 h 22 N 4 o 3 S 3 ,[M+H] + m / z: H Mass: 523.0932. Found: 523.0931.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com