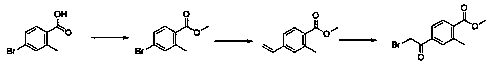
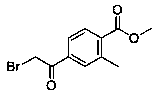
Preparation method of methyl 4-bromoacetyl-2-methylbenzoate
A technology for methyl methyl benzoate and methyl benzoic acid, applied in the field of preparation of methyl 4-bromoacetyl-2-methyl benzoate, meeting the requirements of simple equipment and experimental conditions, mild reaction conditions, and low raw material cost cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] Preferred examples of the invention will be described in detail below. The examples given are to better understand the content of the invention, and the content of the invention is not limited to the examples. Non-essential improvements and adjustments to the embodiment according to the content of the invention still belong to the scope of the invention.
[0026] Synthesis of the first intermediate compound
[0027] In a 500ml one-necked flask, add 4-bromo-2-methylbenzoic acid (20g, 9.3mmol), add methanol 160g, add concentrated sulfuric acid 2ml, heat and reflux for 6 hours, TLC detects that the reaction is complete, add hydrated ethyl acetate, Extracted twice, dried over anhydrous sodium sulfate, and spin-dried to obtain the first intermediate compound (methyl 4-bromo-2-methylbenzoate) as a white solid.
[0028] Synthesis of the second intermediate compound
[0029] In a 500ml single-necked flask, add methyl 4-bromo-2-methylbenzoate (15g, 6.6mmol), 90g of tetrahydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com