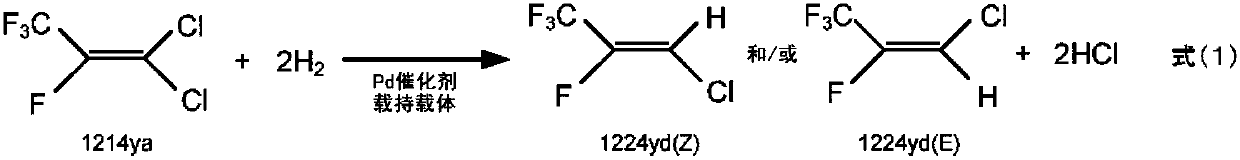
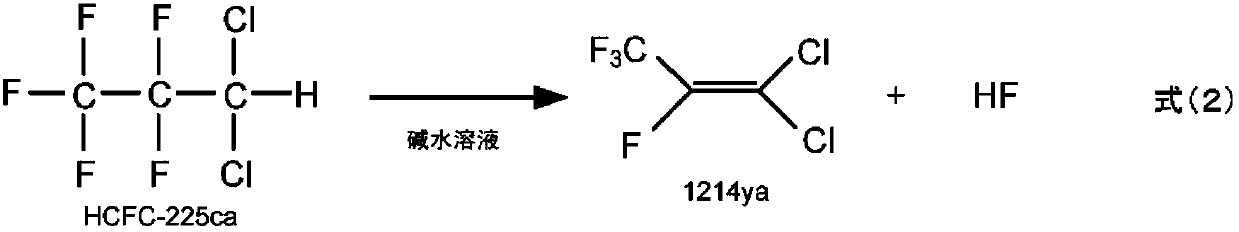
Method for producing 1-chloro-2,3,3,3-tetrafluoropropene
A manufacturing method and technology of tetrafluoropropene are applied in the directions of organic chemistry method, dehalogenation preparation, organic chemistry, etc., to achieve the effect of excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
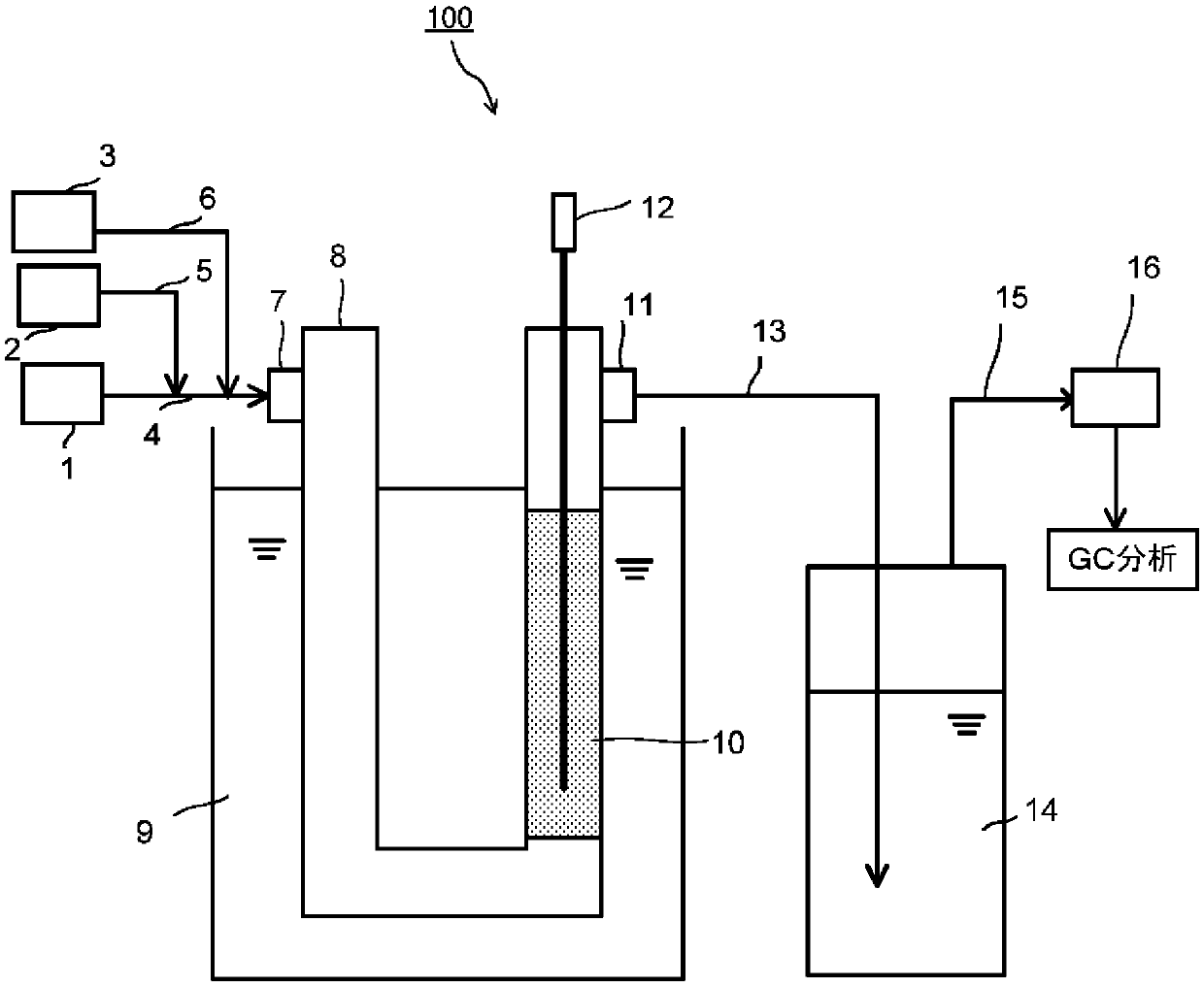
[0104] Examples and comparative examples are shown below to describe the present invention in detail. However, the present invention is not limited by the following description. Examples 1-4 are examples, and Examples 5-6 are comparative examples.
[0105] First, palladium catalyst-supporting carriers used in each example were prepared as follows. Palladium catalyst carriers (X1) and (X2) are palladium catalyst carriers of the present invention, and palladium catalyst carrier (Cf1) is a palladium catalyst carrier used in Comparative Example. In addition, in the preparation of each palladium catalyst carrier, a palladium-supported activated carbon (N.E.chemcat Co., Ltd. (Eヌ. イーケムカャット Co., Ltd.; hereinafter referred to as "palladium supported activated carbon (A)".).
[0106] In addition, in order to calculate the Cl / Pd of the palladium catalyst supporting carrier, the measurement of the above-mentioned (ii) and (iii) is carried out, and [Cl / Pd] is obtained by the formula (3)....
preparation example 1
[0109] Palladium-carrying activated carbon (A) was filled in the reaction tube of the same apparatus as the following reaction apparatus. While maintaining the temperature of the oil bath in which the reaction tube was immersed at 45° C., hydrogen chloride was passed through the palladium-supported activated carbon (A) at a flow rate of 300 mL / sec for 2 hours to obtain a palladium catalyst-carrying carrier (X1). Cl / Pd of the palladium catalyst in the obtained palladium catalyst supporting carrier (X1) calculated by the above calculation method was 2.2.
preparation example 2
[0111] A palladium catalyst-carrying carrier (X2) was obtained in the same manner as in Preparation Example 1, except that the hydrogen chloride treatment time in Preparation Example 1 was changed from 2 hours to 8 hours. Cl / Pd of the palladium catalyst in the obtained palladium catalyst supporting carrier (X2) calculated by the above calculation method was 4.6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com