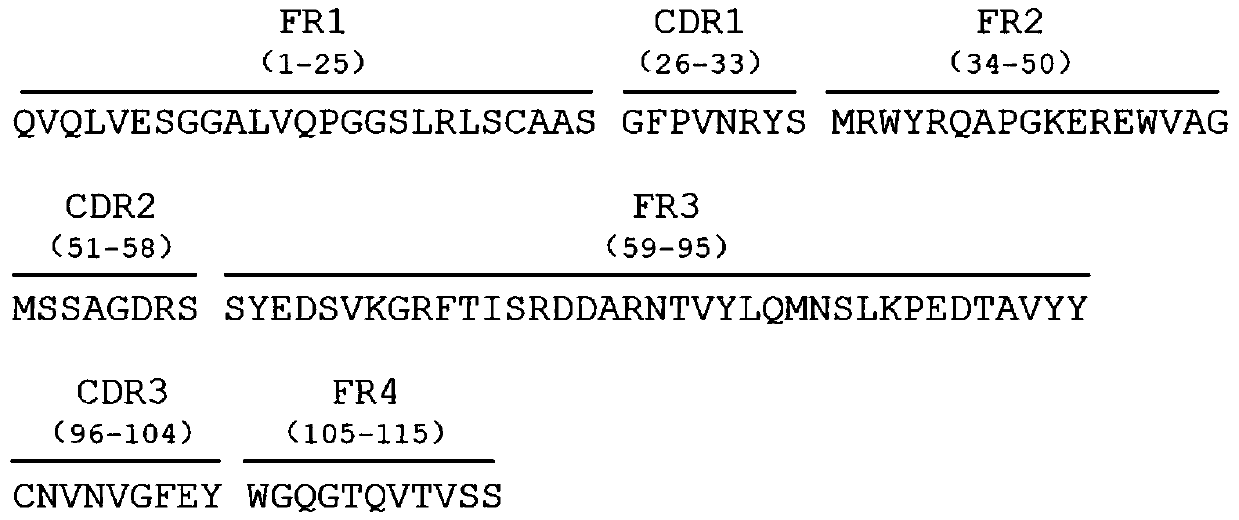
Anti-HET-2 heavy chain antibody and application thereof
A HER-2 and heavy chain antibody technology, applied in the field of molecular immunology, can solve the problems of cumbersome monoclonal antibody development and production process, low specificity and instability of polyclonal antibodies, and achieve excellent gene resources and antibody resources , strong affinity, reliable source effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Panning of Anti-HER-2 Nanobodies
[0028] The single domain heavy chain antibody against HER-2 was panned from the single domain heavy chain antibody phage display library by solid phase affinity panning. Dilute the HER-2 protein with 1×PBS solution to 30-100μg / μL, coat it on the ELISA plate, add 100μL to each well, and coat overnight at 4°C; suck out the coating solution, wash the plate 3 times with PBS, and add to each well 300 μL 4% skimmed milk, block at 37°C for 2 hours; wash the plate with PBS three times and add the phage display library (containing about 1×10 12 CFU), incubated at 37°C for 1 h; aspirate unbound phages, wash the plate 5 times with PBS (increase the number of washes for each round, and the number of washes for each round is shown in Table 1), wash the plate 3 times with PBST, and then add 100 μL wash Remove the liquid (glycine-hydrochloric acid solution, pH 2.2) to elute the phage adsorbed in the plate wells, incubate at 37°C for 5 minu...
Embodiment 2
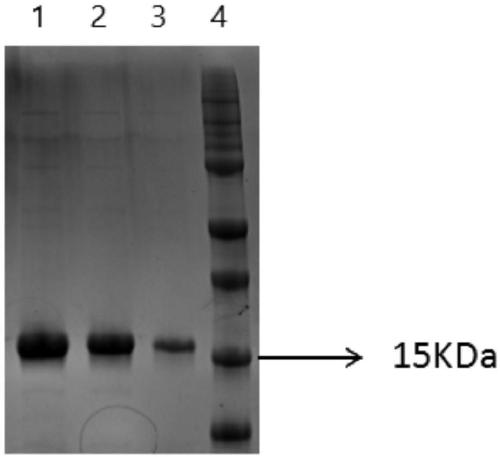
[0036] Example 2 Expression and Purification of Anti-HER-2 Nanobodies
[0037] The gene of the Nanobody obtained in Example 1 was cloned into the expression vector pET-25b (cloned by Qingke Biological Company), and an anti-HER-2 Nanobody expression plasmid was constructed. The constructed expression plasmid was transformed into Escherichia coli BL21, and a single clone was picked for induced expression. Inoculate the single clone into 1L LB liquid medium (containing 100 μg / mL ampicillin) for culture, shake culture at 37°C, 220rmp / min until the OD600 of the bacterial solution reaches 0.5, add IPTG with a final concentration of 0.1mM, and grow at 16°C, 80rmp / min min overnight induction culture. After the culture was completed, the cells were collected by centrifugation, resuspended in 50 mL PBS solution, and then ultrasonically disrupted on ice at 200w for 3 seconds with an interval of 4 seconds for a total of 30 minutes. The supernatant was collected by centrifugation at 8000g...
Embodiment 3
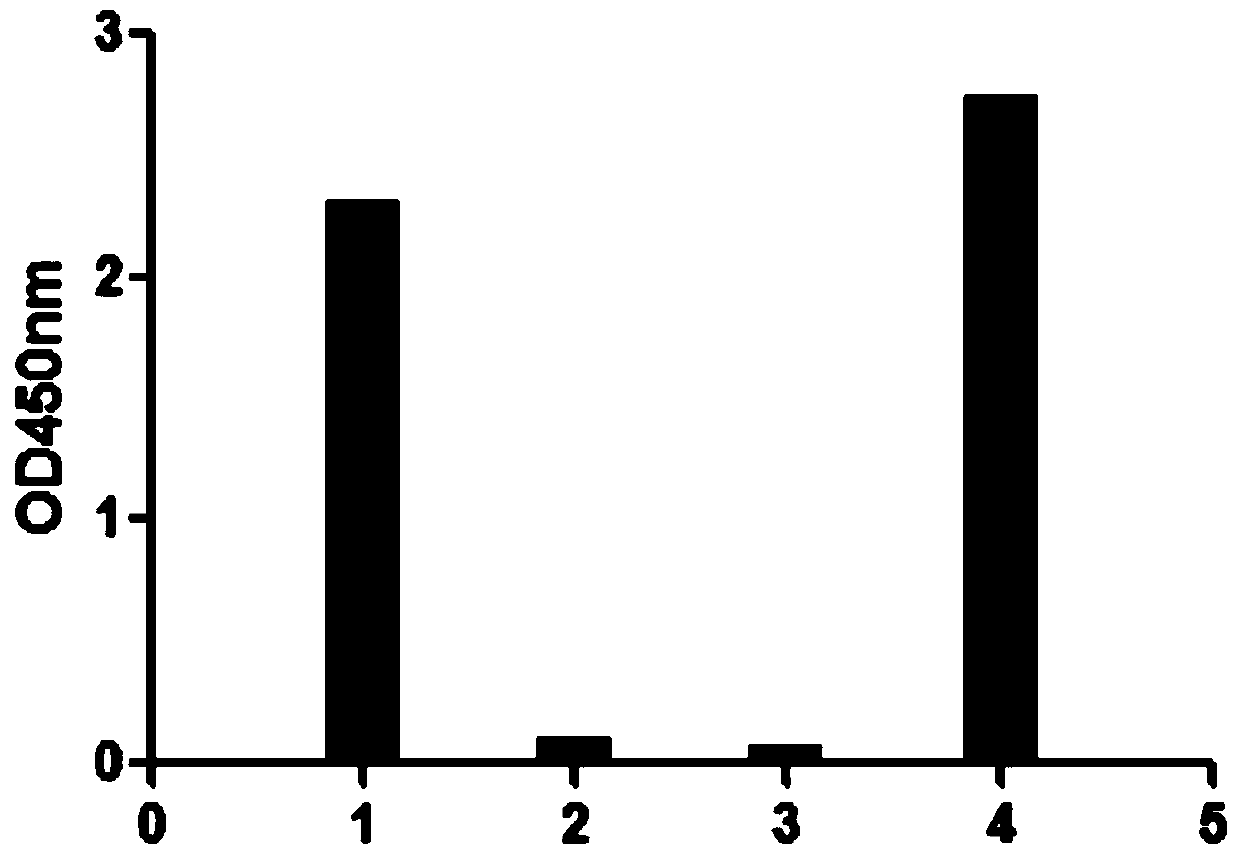
[0039] Example 3 Affinity Determination of Anti-HER-2 Nanobodies
[0040] The Octet@RED96 intermolecular interaction detection system (ForteBio Company) was used to measure the affinity of the Nanobody prepared in Example 2. The Octet@RED96 intermolecular interaction detection system is based on biofilm laminography interferometry (BLI) technology, which can measure the interaction between proteins and biomolecules with only a small amount of sample and without labeling.
[0041] The human HER-2 protein was diluted to 10 μg / mL, and the nanobody obtained in Example 2 was diluted to 50 μg / mL. The diluent used was PBS+0.1% Tween 20+0.1% BSA, and the samples were loaded according to Table 3. Affinity detection chart see Figure 4 .
[0042] Table 3 Affinity detection sample loading table
[0043]
[0044] Equilibrium dissociation constant (affinity) K D (M)=k dis (1 / s) / k on (1 / Ms)
[0045] It was detected that:
[0046] k dis (1 / s) = 0.0004768;
[0047] k on (1 / Ms) =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com