Silanized polymer and preparation method and application thereof
A technology of silylation and polymer, which is applied in the field of silylation polymer and its preparation, can solve the problems of increased viscosity, high reactivity of amino group and NCO group, gelation, etc., achieve high end-capping rate and controllable reaction process , the effect of high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
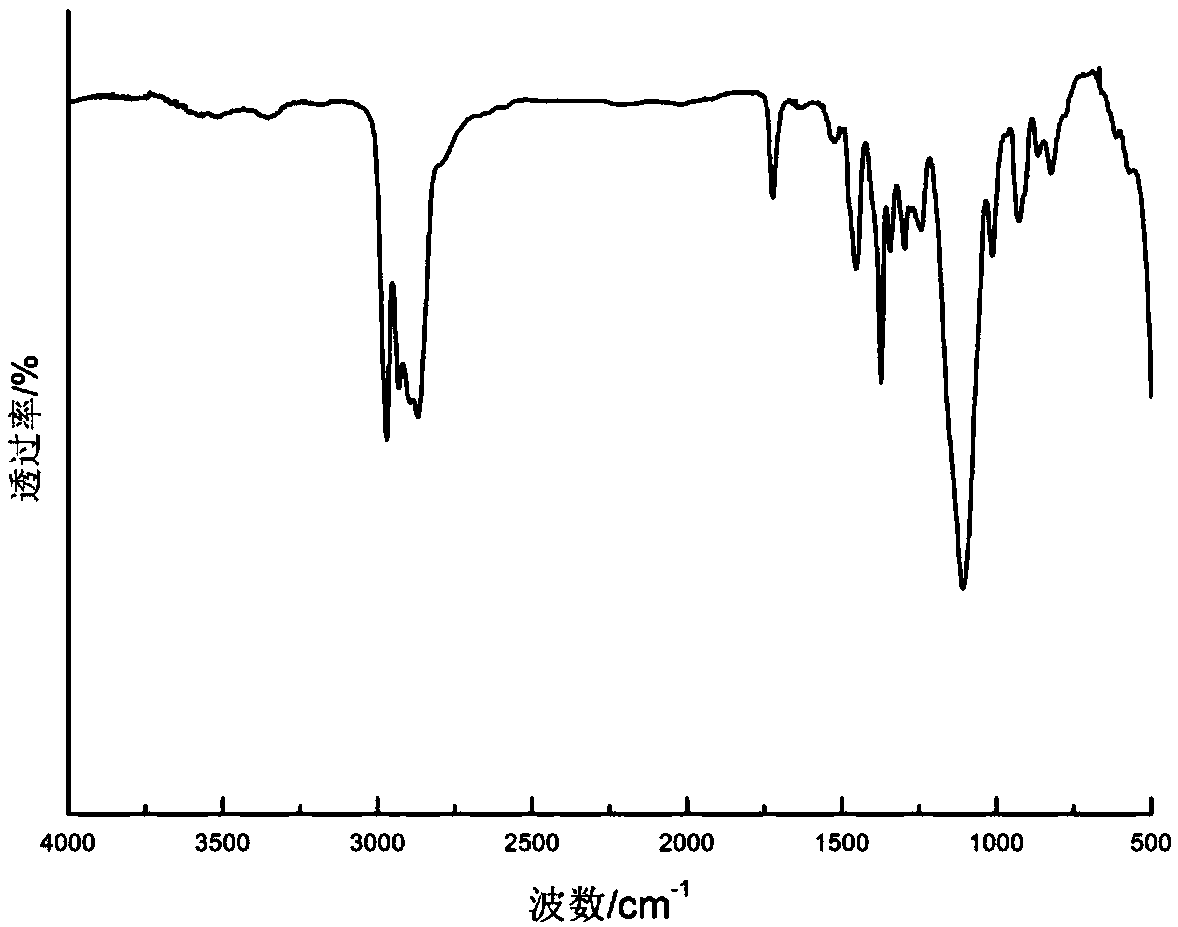
[0080] Add 748.2g of monoallyl polyether alcohol (m=85, n=0, molecular weight about 4988) (0.15mol) to a 2L four-necked flask with a thermometer inserted, at 120°C, -0.090Mpa~-0.1Mpa vacuum Dehydration under pressure for 2h. After cooling down to room temperature, add 2.3g of 5000ppm (based on platinum content) Castel catalyst, stir and mix for 10-15min, heat up to 80°C, add dropwise 23.1g of trimethoxyhydrosilane (95% purity, 0.18mol), The reaction was stirred for 6-8 hours, and the slight excess of organosiloxane was removed under reduced pressure. Under the protection of inert gas, add 0.16g of dibutyltin dilaurate to the four-necked flask, mix and stir evenly, heat up to 80°C, add 20.96g (0.08mol) H 12 MDI was kept at 80°C for 5 hours to obtain the silylated polymer of the present invention.
[0081] The structure of the product was detected by infrared spectroscopy, and the analysis spectrum was as follows figure 1 , 2200cm -1 The near-NCO stretching vibration peak di...
Embodiment 2
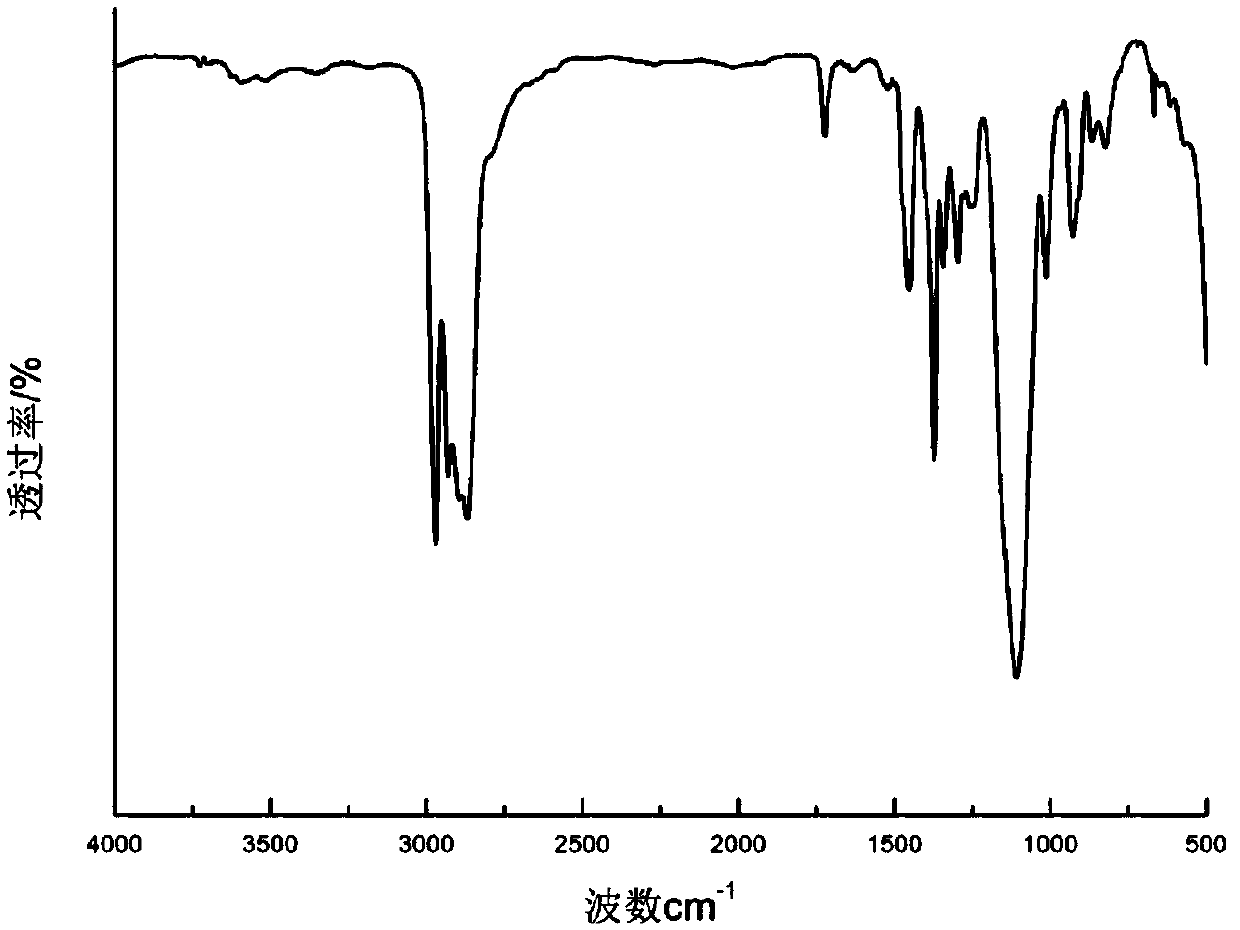
[0085] Add 864.2g of monoallyl polyether alcohol (m=148, n=0, molecular weight about 8642) (0.10mol) into a 2L four-necked flask with a thermometer inserted, at 120°C, -0.090Mpa~-0.1Mpa vacuum Dehydration under pressure for 2h. After cooling down to room temperature, add 5.3g of 5000ppm (based on platinum content) Castel catalyst, stir and mix for 10-15min, heat up to 85°C, add dropwise 15.41g of trimethoxyhydrosilane (95% purity, 0.12mol), The reaction was stirred for 6-8 hours, and the slight excess of organosiloxane was removed under reduced pressure. Under the protection of inert gas, add 0.18g of dibutyltin dilaurate to the four-necked flask, mix and stir evenly, raise the temperature to 90°C, add 13.36g (0.051mol) H 12 MDI was kept at 90°C for 5 hours to obtain the silylated polymer of the present invention.
[0086] The structure of the product was detected by infrared spectroscopy, and the analysis spectrum was as follows image 3 As shown, 2200cm -1 The near-NCO s...
Embodiment 3
[0090] Add 864.2g of monoallyl polyether alcohol (m=148, n=0, molecular weight about 8642) (0.10mol) into a 2L four-necked flask with a thermometer inserted, at 120°C, -0.090Mpa~-0.1Mpa vacuum Dehydration under pressure for 2h. After cooling down to room temperature, add 5.3g of 5000ppm (based on platinum content) Castel catalyst, stir and mix for 10-15min, heat up to 90°C, add 12.85g of methyl dimethoxysilyl hydrogen (95% purity, 0.12 mol), stirred and reacted for 6-8 hours, and the slight excess of organosiloxane was removed under reduced pressure. Under the protection of inert gas, add 0.36g of dibutyltin dilaurate to the four-necked flask, mix and stir evenly, heat up to 80°C, add 11.34g (0.051mol) isophorone diisocyanate (IPDI), and keep warm at 90°C for reaction 5h to obtain the silylated polymer of the present invention.
[0091] The structure of the product was detected by infrared spectroscopy, and the analysis spectrum was as follows Figure 4 As shown, 2200cm -1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com